Despite advances in treatment, cardiovascular disease is the second leading cause of death in Spain. The objective of this study was to determine the cost-effectiveness of the CNIC-Polypill strategy (acetylsalicylic acid 100 mg, atorvastatin 20/40 mg, ramipril 2.5/5/10 mg) compared with the same separate monocomponents for the secondary prevention of recurrent cardiovascular events in adults in Spain.
Materials and methodsA Markov cost-utility model was adapted considering 4 health states (stable, subsequent major adverse cardiovascular event, subsequent ischemic stroke and death) and the SMART risk equation over a lifetime horizon from the perspective of the Spanish National Healthcare System. The CNIC-Polypill strategy was compared with monocomponents in a hypothetical cohort of 1000 secondary prevention patients. Effectiveness, epidemiological, cost and utilities data were obtained from the NEPTUNO study, official databases and literature. Outcomes were costs (in 2021 euros) per life-year (LY) and quality-adjusted LY (QALY) gained. A 3% discount rate was applied. Deterministic one-way and probabilistic sensitivity analyses evaluated the robustness of the model.
ResultsThe CNIC-Polypill strategy in secondary prevention results in more LY (13.22) and QALY (11.64) gains at a lower cost than monocomponents. The CNIC-Polypill is dominant and saves є280.68 per patient compared with monocomponents. The probabilistic sensitivity analysis shows that 82.4% of the simulations are below the threshold of є25,000 per QALY gained.
ConclusionsThe CNIC-Polypill strategy in secondary cardiovascular prevention is cost-effective compared with the same separate monocomponents, resulting in a cost-saving strategy to the Spanish National Healthcare System.
A pesar de los avances en el tratamiento, la enfermedad cardiovascular es la segunda causa de muerte en España. El objetivo de este estudio fue determinar el coste-efectividad de la estrategia CNIC-Polipíldora (ácido acetilsalicílico 100 mg, atorvastatina 20/40 mg, ramipril 2,5/5/10 mg) comparada con los mismos monocomponentes por separado para la prevención secundaria de eventos cardiovasculares recurrentes en adultos en España.
Materiales y métodosSe adaptó un modelo Markov considerando 4 estados de salud (estable, evento cardiovascular adverso mayor posterior, ictus isquémico posterior y muerte) y la ecuación de riesgo SMART con un horizonte temporal de toda la vida desde la perspectiva del Sistema Nacional de Salud español. La estrategia CNIC-Polipíldora se comparó con monocomponentes en una cohorte hipotética de 1000 pacientes en prevención secundaria. Los datos de efectividad, epidemiológicos, de costes y de utilidades se obtuvieron del estudio NEPTUNO, de bases de datos oficiales y de la literatura. Los resultados fueron los costes (en 2021 euros) por año de vida (AV) ganados y AV ajustados por calidad (AVAC) ganados. Se aplicó una tasa de descuento del 3%. Se realizó análisis de sensibilidad univariantes determinísticos univariantes y probabilísticos para evaluar la solidez del modelo.
ResultadosLa estrategia CNIC-Polipíldora en prevención secundaria, produce más ganancias de AV (13,22) y AVAC (11,64) a un coste inferior que los monocomponentes. La CNIC-Polipíldora es dominante y ahorra 280,68 euros por paciente en comparación con los monocomponentes.
El análisis de sensibilidad probabilístico muestra que el 82,4% de las simulaciones están por debajo del umbral de 25000 euros por AVAC ganado.
ConclusionesLa estrategia CNIC-Polipíldora, en prevención cardiovascular secundaria es coste-efectiva en comparación con los mismos monocomponentes por separado, resultando una estrategia que ahorra costes para el Sistema Nacional de Salud español.
Mortality associated to atherosclerotic cardiovascular diseases (ACVD) has been significantly reduced in recent decades mostly due to improvements in prevention, early diagnosis and better treatment.1,2 However, population aging and the increasing frequency of comorbidities rise the risk of suffering recurrent major adverse cardiovascular events (MACE).3,4 18.3% of patients who survive an index acute myocardial infarction (MI) suffer a second cardiovascular (CV) event in the first year,5 and approximately 50% of major coronary events occur in those with a previous hospital discharge diagnosis of acute MI.5
In Spain, ACVD remains one of the major cause of morbidity and mortality and is an uppermost public health problem similarly to other European countries and worldwide.6,7 In 2021, ischaemic heart disease (6.40%) was the second leading cause of death in Spain.7 Additionally, Spain has been characterised by some social inequities in the prevalence of CV risk factors, with wide gender and regional heterogeneity.8 Spain’s 2015 per capita expenditure in CV disease (CVD) was є199 with a total healthcare cost of є9.24 billion.9
Secondary CVD prevention has markedly improved in the last decades1,2 after identifying and more effectively controlling risk factors.10,11 Low-dose acetylsalicylic acid (ASA), statins, and antihypertensive drugs including angiotensin-converting enzyme inhibitors are well-established agents for the prevention of CV events.1,12,13 Patients who consistently take these medications combined in a single pill have a significantly reduced risk of subsequent ACVD events and CV death compared with those who take only one or two of these drugs.14
Previous clinical trials suggested that a treatment strategy based on the CNIC-Polypill (from its name in Spanish, Centro Nacional de Investigaciones Cardiovasculares) improves the control of CV risk factors and reduces the incidence of recurrent ACVD events and death.15–19 In Spain, the Real World Data NEPTUNO (retrospective observational study) showed that CV risk factors control significantly improved with the use of the CNIC-Polypill strategy compared to separate monocomponents (SM) in usual care of clinical practice.3 Additionally, the CNIC-Polypill strategy improves adherence, persistence and patient satisfaction compared to SM-based therapy,20 resulting in improved compliance and greater clinical effectiveness.21,22
Based on adherence, Spanish previous study has shown the CNIC-Polypill strategy to be cost-effective, compared with SM.22 However, that study did not consider the risk factors reduction in secondary prevention with a history of ACVD. Therefore, the aim of this study was to estimate the cost-effectiveness analysis (CEA) of the CNIC-Polypill strategy compared with the SM for preventing recurrent MACE (MI, angina pectoris [AP], transient ischaemic attack [TIA] or peripheral artery disease [PAD]) and ischemic stroke (IS) events because of reducing risk factors in secondary prevention adults in Spain.
MethodsModel designA Markov model was adapted to Spanish National Healthcare System from a previously published CEA developed for Portugal,23 to compare the effectiveness and the costs of the CNIC-Polypill strategy (ASA 100 mg, atorvastatin 20/40 mg, ramipril 2.5/5/10 mg) with the SM to prevent recurrent events in patients with a history of ACVD over the lifetime.
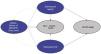
The Markov model considered four mutually exclusive health states, in secondary prevention (Fig. 1): 1) “stable”: previous IS, MACE or both; 2) “subsequent MACE”: new MI, AP, TIA or PAD; 3) “subsequent IS”; 4) death, due to ACVD or to other causes. All patients enter the model in the “stable” state. From the stable state, patients may remain stable, suffer a nonfatal “subsequent” MACE, IS or die. Similar transitions between health states or death could happened at each cycle.
The duration of cycles was 3 months for the first year, to reflect the high risk of recurrence that exists after the index event during this period.24 Thereafter, 1-year-long cycles were considered, in line with other CEA published for ACVD.25 An annual discount rate of 3% to health and cost outcomes was applied following recommendations in Spain.26–28
Transition probabilities through the different health states for the CNIC-Polypill strategy and treatment with SM, respectively, were derived from CV event rates determined by the Secondary Manifestations of Arterial Disease (SMART) risk equation incorporated in the model. The SMART risk equation was developed to predict the ten year probability of recurrent MACE or IS in a population of patients with previous ACVD in a European countries and has been externally validated in multiple regions.29 The SMART risk equation is based on age, gender, and several clinical parameters in a Cox proportional hazards model that calculates the occurrence of MACE and IS (Appendix, equation A1).
The ten-year probability (S0) risk estimated with the SMART29 equation was adjusted to the one-year length of cycles in the model assuming a constant rate; except for the first year which was adjusted to three months. At each cycle, patients’ age was recalculated while the remaining CV risk factors were considered stable throughout the model time horizon due to lack of appropriate time-series data. The coefficients used in the SMART equation are described in the Appendix table A1. The non-cardiac death probability was calculated from mortality tables for men and women in 2019 in Spain.7 Health outcomes were life-years (LY) and quality-adjusted life years (QALYs) gained. Costs were expressed as the total costs of treatments per patient. In addition, incremental cost-effectiveness and incremental cost-utility ratios were obtained.
Data sourcesA literature review of national and international publications was conducted to gather data on the epidemiology, patient characteristics, ACVD usual management, healthcare resources utilization and utility values. The databases consulted were Medline/PubMed, EMBASE, and MEDES. The search strategy is described in the Appendix table A2. All data were validated by a Spanish clinical expert in managing secondary prevention ACVD patients.
The Spanish Ministry of Health [RAE-CMBD]30 and the General Council of Pharmaceutical Associations databases [Botplus]31 were searched to obtain the unitary costs of the acute CV events and drugs, respectively.
Patient populationA hypothetical cohort of 1000 patients with a history of either IS, MACE or both was simulated. This cohort had the baseline characteristics of the Spanish population with a previous ACVD, according to previously published observational studies (Appendix table A3).42–50
EffectivenessEffectiveness data was derived from NEPTUNO3 cohort study that investigated the clinical effectiveness (control of CV risk factors and incidence of recurrent MACE), resource use, and healthcare costs of the CNIC-Polypill strategy compared with the combination of SM or therapeutic equivalents in routine clinical practice for secondary prevention in Spanish adult population.
For the base case of this analysis, cohort 2 (identical SM) was selected as the comparator to closely compare the CNIC-Polypill strategy against the SM. The following CNIC-Polypill effectiveness rates compared to SM were applied: 1.80% reduction for systolic blood pressure, 5.28% for total cholesterol and 4.01% increase for high-density lipoprotein cholesterol.3
Costs and health utilitiesOnly direct medical cost were consider: fatal and non-fatal events cost, post-event follow up cost and drugs costs (Table 1 and Appendix table A4).7,22,30,30,31 Costs were reported in 2021 euros. If necessary, cost were updated to 2021 euros using the medicine consumer price index.7 Utility values for each health state are summarized in Table 1.32
Costs and utilities.
| Costs ± SE | Health utilities ± SE | |||
|---|---|---|---|---|
| Value | Source | Value | Source | |
| Acute events | ||||
| MACE (non-fatal) | є6,288.3 ± є1,257.7 | 7,30 | 0.76 ± 0.0.152 | 32 |
| MACE (fatal) | є12,025.8 ± є2,405.2 | 7,30 | 0.00 ± 0.000 | 32 |
| IS (non-fatal) | є5,204.0 ± є1,040.8 | 7,30 | 0.63 ± 0.126 | 32 |
| IS (fatal) | є7,473.1 ± є1,494.6 | 7,30 | 0.00 ± 0.000 | 32 |
| Death | є0.0 ± є0.0 | 0.00 ± 0.000 | 32 | |
| Follow-up post-events | ||||
| post-MACE | є598,1 ± є119,6 | 7,30 | 0.84 ± 0.167 | 32 |
| post-IS | є4.288,4 ± є857,7 | 7,30 | 0.69 ± 0.138 | 32 |
IS: Ischemic Stroke; MACE: mayor adverse cardiovascular event; SE: standard error.
Deterministic one-way (OWA) and probabilistic (PSA) sensitivity analyses were performed to assess the influence of the uncertainty of parameters on the robustness of the model and its results. In the OWA, different assessments were made based on the possible variation of the most sensitive parameters: basal average age, eGFR parameter, discount rate, costs of acute MACE, mortality rates and utility values. These parameters were varied according to the standard error or assuming ±20% modification in the absence of variability information in the base case data. The results of the OWA were represented in a tornado diagram.
The PSA was performed in a Monte-Carlo simulation of 1000 iterations based upon model inputs randomly drawn from distributions around the mean.33 Normal distribution for the population demographics and clinical data including all CV risk equations (except risk of recurrent IS); beta distribution for rates, percentages, effectiveness and utility data, and gamma distribution for costs were applied.33 A lognormal distribution was employed for the relative risk of recurrent IS. The PSA results were represented in the cost-effectiveness plane and the acceptability curve.
ResultsBase case scenarioTable 2 shows the main results of the base case analysis. For a simulated cohort of 1000 secondary prevention patients, the CNIC-Polypill strategy avoids 67 ACVD events (61 MACE and 6 IS events) over the cohort lifetime. Compared to the cohort on the SM, the cohort on the CNIC-Polypill strategy gains a total of 13.22 discounted LY and a total of 11.64 discounted QALYs per 1000 patients over a lifetime.
Incremental Cost-Effectiveness Ratio results in the base case scenario.
| CNIC-Polypill | Monocomponents | CNIC-Polypill vs. Monocomponents | |
|---|---|---|---|
| Costs outcomes | |||
| Total direct costs | є41,870,812 | є42,151,487 | -є280,675 |
| Drug costs | є2,316,137 | є2,213,752 | -є102,386 |
| Management cost, acute ACVD event: | є6,684,191 | є7,047,549 | -є363,358 |
| Subsequent non-fatal MACE | є5,815,418 | є6,131,548 | -є316,131 |
| Subsequent fatal MACE | є250,176 | є263,776 | -є13,600 |
| Recurrent non-fatal IS | є520,866 | є549,181 | -є28,315 |
| Recurrent fatal IS | є97,731 | є103,042 | -є5,312 |
| Management cost, follow-up ACVD event*: | є32,870,484 | є32,890,186 | -є19,703 |
| Health outcomes | |||
| Subsequent non-fatal MACE | 1,031.71 | 1,092.36 | −60.65 |
| Subsequent non-fatal IS | 113.17 | 119.32 | −6.15 |
| Subsequent fatal MACE | 33.88 | 35.72 | −1.84 |
| Subsequent fatal IS | 562.67 | 561.27 | 1.39 |
| LY | 12,717.25 | 12,704.03 | 13.22 |
| QALYs | 9,705.36 | 9,693.73 | 11.64 |
| ICER (є per LY gained) | DOMINANT | ||
| ICUR (є per QALY gained) | DOMINANT | ||
ACVD: atherosclerotic cardiovascular diseases; CNIC: from its name in Spanish, Centro Nacional de Investigaciones Cardiovasculares; ICER: incremental cost-effectiveness ratio; ICUR: incremental cost-utility ratio; IS: Ischemic Stroke; LY: life year; MACE: mayor adverse cardiovascular event; QALY: quality-adjusted life year.
The total discounted costs for the CNIC-Polypill strategy cohort is є41,870,812 compared with є42,151,487 for the cohort on the SM. The total discounted incremental savings amounts to є280,675 with the CNIC-Polypill strategy. Thus, the CNIC-Polypill strategy is dominant being less costly and providing additional health benefits compared with the SM. In addition, the CNIC-Polypill strategy still being a dominant alternative for secondary ACVD prevention regardless gender (Appendix table A4 and table A5).
Sensitivity analysisOWA analysisFigure A1 in the Appendix shows the results of the OWA (tornado diagram). Mean cohort age is the input parameter with the greatest impact on results, being the only one with no dominant result in this analysis.
PSA analysisThe PSA demonstrates the robustness of the CEA results obtained in the base case scenario. The cost-effectiveness plane (Fig. 2) shows that the CNIC-Polypill strategy is costs-saving in 84.0% of the simulations and provides greater health benefits in 91.0% of the simulations compared with the SM. Likewise, 78.0% of the simulations are dominant. The acceptability curve (Fig. 3) shows that in 82.4% of the simulations, the CNIC-Polypill strategy is cost-effective at the recommended Spanish threshold of є25,000/QALYs.34,35
DiscussionThe present CEA evaluated the potential health outcomes and cost benefits of a therapeutic approach based on the CNIC-Polypill strategy vs SM for the ACVD secondary prevention from the perspective of the Spanish National Healthcare System. In the base case scenario and in a cohort of 1000 patients, almost seventy non-fatal CV events can be avoided, and more than ten extra LY and QALYs can be gained over the patients’ lifetime at a lower cost with the CNIC-Polypill strategy than usual care using the SM. The CNIC-Polypill strategy is, therefore, a dominant therapeutic approach in real clinical practice in Spain.
These findings are in line with the results of the reference model published for Portugal.23 Based on improving blood pressure and lipid profile control and on reducing CV risk of recurrent events post-MACE and post-IS,3 the CNIC-Polypill strategy significantly reduces new events and increases the length and quality of life in secondary prevention population in both countries. Both, in Spain and in Portugal, willingness-to-pay estimates and sensitivity analyses indicate that the CNIC-Polypill strategy is consistently cost-effective and largely affordable compared with the monocomponents based-therapy. These results remain consistent despite different secondary prevention patients risk profile as demonstrated in the PSA.
Previous CEAs carried out in the United Kingdom21 and in Spain22 had already suggested the health and cost benefits of adopting the CNIC-Polypill strategy compared with monocomponents even when only improvements in treatment adherence were considered.
The effectiveness of the CNIC-Polypill strategy for controlling risk factors in CVD prevention population has been widely demonstrated.15–18,36–38 The latest evidence from a multicentre, multinational clinical trial (SECURE)39 substantiates the validity of the effectiveness data and results estimated in the model. The SECURE39 clinical trial showed that after a median of 3 years of treatment, the primary composite outcome of CV death, nonfatal type 1 MI, nonfatal IS, or urgent revascularization occurred less frequently among patients in the CNIC-Polypill strategy group compared with those in the usual care group.
Likewise, other clinical studies have shown that patients previously receiving equally potent treatment with atorvastatin and ramipril had a greater reduction in cholesterol and systolic blood pressure after initiating treatment with the CNIC-Polypill. A synergistic effect of the components might explain the gains in effectiveness found with the CNIC-polypill strategy.40,41 These benefits could favour secondary ACVD prevention in men and women alike, as suggested by the similar incremental cost-effective results found in the model.
A key contribution of this CEA study is the incorporation of the risk factors in the SMART CV risk equation to estimate transition probabilities for the occurrence of CV events. The CV risk equations increase the predictive power of events in the model, although remains still unclear which is the optimal prediction risk equation.42 Also, a differentiating characteristic is the transition of secondary prevention individuals with a previous MACE or IS event throughout the different health states included in the Markov model.
However, the structural rigidity of all Markov models makes difficult to modelling a pathology as complex as ACVD. This implies a simplification of the real clinical practice and therefore entails a series of limitations. The model’s long-term CV event prediction over a lifetime horizon is subjected to uncertainties linked to the variable individual patients’ characteristics, determining their transition throughout the health states. This variability is not reflected in the model.
In this model, the SMART29 risk equation was considered appropriate. The Framingham risk equation43–46 does not calculate the risk of secondary ACVD making its usage in the model less preferable, despite the existence of an adapted version to the Spanish population.46,47 A similar adaptation of the SMART risk equation to Spain population would increase the precision of risk calculations in both cohorts. The SMART risk equation predicts a composite of recurrent ACVD events, without differentiating between fatal and nonfatal events and does not consider the cumulative risk of patients that have already suffered from recurrent events. To overcome some of the Markov model limitations, sensitivity analyses were performed, including the PSA using the Monte-Carlo simulations.33 In all scenarios, the CNIC-Polypill is cost-effective compared to monocomponents supporting the consistency of results.
Finally, the perspective adopted does not account for the losses in productivity or for the disability that ACVD may imply for the Spanish society neither consider other aspects relevant to CVD care and prevention such as regionality or premature death, among other that may be important in the current organizational, epidemiological and demographic context of Spain.8,48,49
Besides these limitations, the results of the pharmacoeconomic model show that the use of the CNIC-Polypill strategy in secondary prevention patients with a history of MACE or IS is cost-effective compared with the SM.
ConclusionsOverall, this CEA shows the potential health and economic achievements to be accomplished from better controlling CV risk factors with the CNIC-Polypill strategy as baseline therapy in secondary cardiovascular prevention in real clinical practice in Spain. It suggests that the CNIC-Polypill strategy is a highly convenient approach to significantly reduce risk factors and recurrent MACE and IS in secondary ACVD prevention, compared with the SM. The CNIC-Polypill strategy is affordable, cost-effective and cost-saving in the Spanish Healthcare setting.
FundingThis work was supported by Ferrer.
Conflicts of interestThe authors declare the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: AGD and AD are employees of Weber and received funds from Ferrer to develop this study. AHV has no conflicts of interest. VB received honoraria from Ferrer for validation and review of the data and manuscript.
The authors would like to thank Martin Hopmans, Ester Fernandez and Aránzazu Real, from Ferrer Internacional, for their critical review, Silvia Paz, from Smartworking4u, for her critical review of the English translation, and Carlos Dévora and Mathilde Daheron, from Weber, for their help in reviewing the literature.