The aim of this study was to assess the available evidence on the validity, diagnostic accuracy and clinical utility of the multitarget DNA test in feces (Cologuard™) for screening for colorectal cancer (CRC).
Material and methodsA systematic review was performed by consulting MedLine, EMBASE and Web of Science to July 2014. Studies on diagnostic tests were selected that evaluated the test in asymptomatic adults who underwent CRC screening. The quality and risk of bias were assessed using the Quality Assessment of Diagnostic Accuracy Studies tool. The level of evidence was defined according to the National Institute for Health and Clinical Excellence. A qualitative synthesis was conducted.
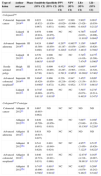
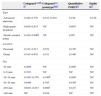
ResultsA total of 299 literature references were identified, including 1 synthesis report and 5 diagnostic test studies. Three of the five studies had a case-control design in Sackett phase II and were of moderate quality, and two had a prospective design in Sacket phase III and were of high quality. The sensitivity for detecting CRC was greater than 90%, but only 40% for detecting advanced adenomas. The test provided conclusive diagnostic evidence to rule out CRC (negative likelihood ratio, LR−: 0.02–0.09), although it was not useful for ruling out advanced adenoma (LR−: 0.5–0.7).
ConclusionsThe Cologuard™ test is a valid screening test for ruling out cancerous lesions but is suboptimal for ruling out precancerous lesions. There is no evidence in terms of mortality, survival or cost-effectiveness.
El objetivo fue evaluar la evidencia disponible sobre la validez, precisión diagnóstica y utilidad clínica del test multidiana de ADN en heces (Cologuard™) en el cribado de cáncer colorrectal (CCR).
Material y métodosSe realizó una revisión sistemática consultando MedLine, EMBASE y Web of Science hasta julio de 2014. Se seleccionaron estudios de pruebas diagnósticas que evaluaran el test en adultos asintomáticos sometidos a cribado de CCR. La calidad y el riesgo de sesgo se evaluaron mediante la herramienta Quality Assessment of Diagnostic Accuracy Studies. El nivel de evidencia se definió según The National Institute for Healthand Clinical Excellence. Se realizó una síntesis cualitativa.
ResultadosSe identificaron 299 referencias bibliográficas, incluyéndose un informe de síntesis y cinco estudios de pruebas diagnósticas, tres de ellos con diseño caso-control en fase II de Sackett y de moderada calidad, y dos con diseño prospectivo en fase III de Sacket y de alta calidad. La sensibilidad para detectar CCR fue superior al 90%, pero solo del 40% para la detección de adenoma avanzado. El test proporcionó evidencia diagnóstica concluyente para descartar CCR (cociente de probabilidad negativo, CPN: 0,02-0,09), aunque no fue útil para descartar adenoma avanzado (CPN: 0,5-0,7).
ConclusionesEl test Cologuard™ es una prueba de cribado válida para descartar lesiones cancerosas, resultando subóptima para descartar lesiones precancerosas. No hay evidencia sobre resultados en términos de mortalidad o supervivencia, ni sobre coste-efectividad.
Article
Diríjase desde aquí a la web de la >>>FESEMI<<< e inicie sesión mediante el formulario que se encuentra en la barra superior, pulsando sobre el candado.

Una vez autentificado, en la misma web de FESEMI, en el menú superior, elija la opción deseada.

>>>FESEMI<<<