Chronic obstructive pulmonary disease (COPD) is a common chronic respiratory disease which can cause organ damage and even death. For the first time, the prevalence and risk factors of COPD in Shanxi Province (China) were evaluated in this study.
MethodsA population-based survey was conducted in 2015 based on the Shanxi Province population (age ≥ 20). COPD was diagnosed according to the Global Initiative for Chronic Obstructive Lung Disease (GOLD) standard (2017).
ResultsA total of 5636 participants with reliable post-bronchodilator results were selected. The prevalence of spirometry-defined COPD among the population (age ≥ 20) was 6.4% (95% CI 5.8–7.1) and was more prevalent in men (9.7%, 95% CI 8.6–10.9) than women (3.9%, 95% CI 3.2–4.6). The multivariate-adjusted analysis demonstrated that sex, age, education, smoking, chronic cough during childhood (age ≤ 14), and a family history of parents with respiratory diseases were related to the prevalence of COPD risk. On the contrary, among rural residents living with smokers, a history of pneumonia or bronchitis during childhood, BMI, use of biomass energy, prolonged exposure to particulate matter 2.5, and a family history of parents with respiratory diseases did not show a significant correlation to COPD.
ConclusionsWe have identified a high prevalence of COPD and its determinants in Shanxi province. The prevention of COPD and its early detection is a health priority in this province.
La enfermedad pulmonar obstructiva crónica (EPOC) es un trastorno respiratorio crónico frecuente que puede causar daños orgánicos e incluso la muerte. En este estudio se evalúan por primera vez la prevalencia y los factores de riesgo de la EPOC en la provincia de Shanxi (China).
MétodosEn 2015 se realizó una encuesta poblacional basada en la población de la provincia de Shanxi (edad ≥ 20 años). La EPOC se diagnosticó según el patrón de la Iniciativa Global para la Enfermedad Pulmonar Obstructiva Crónica (GOLD; 2017).
ResultadosSe seleccionó a 5.636 participantes con resultados fiables después del uso de un broncodilatador. La prevalencia de EPOC determinada mediante espirometría en la población (edad ≥ 20 años) fue del 6,4% (IC del 95%, 5,8−7,1) y fue mayor en los hombres (9,7%; IC del 95%, 8,6–10,9) que en las mujeres (3,9%; IC del 95%, 3,2–4,6). El análisis de ajuste múltiple demostró que el sexo, la edad, la educación, el tabaquismo, la tos crónica durante la infancia (edad ≤ 14 años) y los antecedentes de enfermedades respiratorias de los padres de los participantes estaban relacionados con la prevalencia del riesgo de EPOC. En cambio, los antecedentes de neumonía o bronquitis durante la infancia, el índice de masa corporal, el uso de biomasa, la exposición prolongada a partículas PM 2,5 y los padres con antecedentes de enfermedades respiratorias no se relacionaron de forma significativa con la EPOC entre los residentes de las áreas rurales que convivían con fumadores.
ConclusionesHemos identificado una elevada prevalencia de EPOC y sus determinantes en la provincia de Shanxi. La prevención de la EPOC y su detección precoz son prioridades de salud en esta provincia.
Chronic obstructive pulmonary disease (COPD) is a common chronic respiratory disease, which is defined as a progressive and partially reversible respiratory tract disease characterized by airflow restriction, usually caused by chronic bronchitis or emphysema.1
According to the statistics of the Global Burden of Disease Study on COPD, 174.5 million people were suffering from COPD2,3 and 3.2 million died in 2015 worldwide.4 It was also the disease with the fifth highest fatality rate in China in 2016,5 and the morbidity and mortality of COPD has been increasing in recent years, which might be affected by the aging population and environmental pollution in China.6
A survey of COPD in China showed that the prevalence of COPD was about 8.2% in the population over 40 years old (12.4% for men and 5.1% for women) between 2002 and 2004.7 According to the China Pulmonary Health (CPH) reports,8 the prevalence of COPD in the population over 20 years old was 8.6%, amounting to about 99.9 million people. Studies by Wang Chen8 in 2015 (13.7%) reported that the COPD prevalence in the population over 40 years old was significantly higher than that reported in research by Zhong et al.7 from 2008 (8.2%), indicating that COPD morbidity had risen rapidly and reached epidemic rate in China, although the study methods were not completely similar.
The prevalence of COPD differs between different regions of China, which may be affected by the economy, lifestyle, and population demographics.7,9 According to the Population Census in 2015, the population of Shanxi Province was approximately 36 million, of which 78% was over 20 years old,8 and 55% represented the urban population.10 Due to the high smoking rate among Chinese men, there was an urgent need to publish public health policies for specific regions to prevent COPD disease and better allocate health resources. Nevertheless, the prevalence of COPD in Shanxi Province had not been published,9 so the relationship between smoking or other risk factors and COPD prevalence in Shanxi was not clear.
Here, we report for the first time the prevalence of COPD in Shanxi and the risk factors related to COPD.
MethodsSample selectionIn this study, subjects aged 20 years or older were selected through a multistage stratified cluster sampling method from Shanxi Province, China, between June 2012, and May 2015. Firstly, a large city, a midsize city, an economically developed county, and an underdeveloped county (based on average GDP) in Shanxi Province were selected randomly. Secondly, two urban districts from each city, two towns from the developed county, and two towns from the underdeveloped county were randomly chosen. Thirdly, we selected at random individuals aged 20 years or older from these communities. Only one person could be selected from one family without replacement. Lastly, we stratified these participants by age and gender according to the Chinses census data from 2010.10
Only permanent residents who had lived in Shanxi Province for more than one year could be included in this study. In addition, individuals were excluded if they were physically incapable of taking a spirometry test, such as patients with myocardial infarction in the past three months or heart rate of more than 120 beats per minute.
This study was approved by the Ethics Review Committee of Beijing Capital Medical University and other participating agencies, and all participants signed the informed consent.
ProceduresWe obtained survey data from local health centers, and then designed a standardized questionnaire for the participants,8 which included demographic data, medical history, family history of respiratory disease, and other risk factors. People who had smoked more than 100 cigarettes in their lifetime were defined as current smokers, and people who resided with smokers were defined as passive smokers.
Biomass utilization meant that the individuals cooked by wood or animal feces in the past six months. History of childhood pneumonia or bronchitis was defined as admission to hospital at least once before the age of 14, which was further divided into frequent (cumulative >3 months per year), occasional (1–3 months per year), and rare (<1 month per year).
History of chronic bronchitis referred to cough and sputum over at least three months within two years. The exposure to ambient particles with diameter less than 2.5 μm (PM2.5) was obtained by the aerosol optical depth model, which was recovered from the regional satellite.11 The PM 2.5 concentration model (2010) of each selected region was used as the air contamination exposure indicator for the subjects.
The pulmonary function tests were performed by certified technicians using the MasterScreen Pneumo PC spirometer (CareFusion, Yorba Linda, CA, USA), which was calibrated daily using a 3 L syringe. Subjects were asked to perform eight exhalations until 1 s of forced vital capacity (FVC) and forced expiratory volume (FEV1) could be restored within 150 mL.12 The bronchodilator (salbutamol 400 μg) was inhaled with same standard at 500 mL intervals, and then spirometry was performed after 20 min. All participants performed all spirometry measurements, including in a sitting posture, with nose clips, and disposable mouthpiece.
Data quality control was performed according to the American Thoracic Society and the European Respiratory Society standards.
OutcomesBased on the Global Initiative for Chronic Obstructive Lung Disease (GOLD) standard (2017), the ratio of FEV1: FVC of less than 70% after bronchodilator use was defined as COPD.13 The ratio of FEV1: FVC and the obstruction level were predicted based on the general population references in the United States (GOLD 1, ≥80%, GOLD 2, ≥50% to <80%, GOLD 3, ≥30% ∼ <50%).14 The lower limit of normal (LLN) reference values in China were used to analyze the sensitivity of COPD,15 and COPD diagnoses were made by clinicians according to the self-reported spirometry results.
Statistical analysisBased on the survey data, five age groups (20–39 years, 40–49 years, 50–59 years, 60–69 years, and ≥70 years) were designed for both men and women. The sample size was calculated using PASS software (NCSS, Kaysville, UT, USA). The weights and age-standardized prevalence were calculated based on 2010 China census population data. The ANOVA was used to assess the diversities between continuous variables and categorical variables with the χ2 test. A multivariable logistic regression analysis was performed to investigate risk factors for all participants and only nonsmokers, respectively.
All data analysis was conducted with SUDAAN (version 11.0; Research Triangle Institute, Research Triangle Park, NC, USA) and SAS (version 9.4; SAS Institute, Cary, NC, USA).
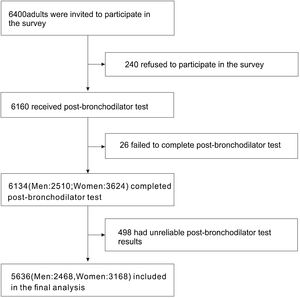
ResultsBasic characteristics of the participantsA total of 6400 individuals were invited to participate in our survey, of which 240 (3.75%) individuals declined to participate. After excluding unreliable results, 5636 cases (2468 men and 3168 women) were included for further analysis (Fig. 1).
According to the survey results, the prevalence of COPD was estimated to be 6.4% (95% CI 5.8–7.1) of the population (age ≥ 20) in Shanxi Province (Table 1 and Supplementary Table 1). The COPD prevalence was also calculated based on each characteristic at different GOLD stages. In male participants, the prevalence of COPD was 6.4%, 2.8%, and 0.4% at GOLD stages I, II, and III, while it was 2.6%, 1.1%, and 0.2% in female, respectively. The prevalence of COPD increased with age, and the prevalence of the 20−39 age group was 1.5% (95% CI 1.0–2.0), which increased to 9.3% (95% CI 8.4–10.3) in the group aged 40 years or older (p < 0.0001 for the age difference) (Tables 2 and 3).
Population characteristics of subjects with COPD.
| Men | Women | |||||||
|---|---|---|---|---|---|---|---|---|
| Total | No COPD | COPD | p value | Total | No COPD | COPD | p value | |
| (n = 2468) | (n = 2228) | (n = 240) | (n = 3168) | (n = 3045) | (n = 123) | |||
| Age (years) | <0.001 | <0.001 | <0.001 | |||||
| 20−39 (%) | 1086 (44.0) | 1067 (98.3) | 19 (1.7) | 992 (31.3) | 980 (98.8) | 12 (1.2) | ||
| 40−49 (%) | 466 (18.9) | 420 (90.1) | 46 (9.9) | 721 (22.8) | 698 (96.8) | 23 (3.2) | ||
| 50−59 (%) | 502 (20.3) | 436 (86.9) | 66 (13.1) | 920 (29.0) | 878 (95.4) | 42 (4.6) | ||
| 60−69 (%) | 343 (13.9) | 264 (77.0) | 79 (23) | 463 (14.6) | 425 (91.8) | 38 (8.2) | ||
| ≥70 (%) | 22 (0.9) | 14 (63.6) | 8 (36.4) | 20 (0.6) | 19 (95.0) | 1 (5.0) | ||
| Resident | <0.419 | <0.241 | ||||||
| Urban residents (%) | 2284 (92.5) | 2065 (90.4) | 219 (9.6) | 2896 (91.4) | 2780 (96.0) | 116 (4.0) | ||
| Rural residents (%) | 184 (7.5) | 163 (88.6) | 21 (11.4) | 272 (8.6) | 265 (97.4) | 7 (2.6) | ||
| Smoking history (pack/years) | <0.001 | <0.001 | ||||||
| 0 (%) | 803 (32.5) | 662 (82.4) | 141 (17.6) | 32 (1.0) | 24 (75.0) | 8 (25.0) | ||
| 1∼9 (%) | 471 (19.1) | 456 (96.8) | 15 (3.2) | 36 (1.1) | 32 (88.9) | 4 (11.1) | ||
| 10∼19 (%) | 310 (12.6) | 282 (91.0) | 28 (9.0) | 18 (0.6) | 16 (88.9) | 2 (11.1) | ||
| ≥20 (%) | 884 (35.8) | 828 (93.7) | 56 (6.3) | 3082 (97.3) | 2973 (96.5) | 109 (3.5) | ||
| Smoking status | <0.01 | <0.001 | ||||||
| Never (%) | 771 (31.2) | 720 (93.4) | 51 (6.6) | 3072 (97.0) | 2963 (96.5) | 109 (3.5) | ||
| Former smoker (%) | 1468 (59.5) | 1314 (89.5) | 154 (10.5) | 72 (2.3) | 63 (87.5) | 9 (12.5) | ||
| Current smoker (%) | 229 (9.3) | 194 (84.7) | 35 (15.3) | 24 (0.8) | 19 (79.2) | 5 (20.8) | ||
| BMI (kg/m2) | 25.16 ± 3.78 | 25.20 ± 3.75 | 22.70 ± 3.07 | <0.001 | 24.61 ± 3.78 | 24.61 ± 3.78 | 26.46 ± 5.02 | <0.001 |
| Symptoms chronic cough during childhood (age ≤14 years) | 0.011 | <0.001 | ||||||
| Frequent (%) | 55 (2.2) | 43 (78.2) | 12 (21.8) | 118 (3.7) | 101 (85.6) | 17 (14.4) | ||
| Sometimes (%) | 181 (7.3) | 163 (90.1) | 18 (9.9) | 214 (6.8) | 204 (95.3) | 10 (4.7) | ||
| Rare (%) | 2225 (90.2) | 2016 (90.6) | 209 (9.4) | 2827 (89.2) | 2731 (96.6) | 96 (3.4) | ||
| History of pneumonia or bronchitis during childhood | p = 0.014 | <0.001 | ||||||
| Yes (%) | 147 (6.0) | 129 (87.7) | 18 (12.3) | 202 (6.4) | 182 (90.1) | 20 (9.9) | ||
| No (%) | 1981 (80.3) | 1805 (91.1) | 176 (8.9) | 2575 (81.3) | 2489 (96.7) | 86 (3.3) | ||
| Unknown (%) | 337 (13.7) | 291 (86.4) | 46 (13.6) | 385 (12.2) | 368 (95.6) | 17 (4.4) | ||
| Parental history of respiratory diseases | 2468 (100) | 2228 (90.3) | 240 (9.7) | p = 0.422 | 3168 (100) | 3045 (96.1) | 123 (3.9) | p = 0.242 |
| History of tuberculosis | p = 0.001 | p = 0.792 | ||||||
| Yes (%) | 42 (17.1) | 36 (85.7) | 6 (14.3) | 77 (2.4) | 75 (97.4) | 2 (2.6) | ||
| No (%) | 2421 (82.9) | 2187 (90.3) | 234 (10.6) | 3087 (97.6) | 2966 (96.1) | 121 (3.9) | ||
| History of spirometry test | p = 1.000 | p = 0.197 | ||||||
| Yes (%) | 2441 (98.9) | 2206 (90.4) | 235 (9.6) | 3131 (98.8) | 3010 (96.1) | 121 (3.9) | ||
| No (%) | 11 (0.4) | 10 (90.9) | 1 (9.1) | 21 (0.7) | 19 (90.5) | 2 (9.5) | ||
| FEV1 L (%) | 3.39 (71.0) | 3.49 (64.0) | 1.06 (17.0) | < 0.001 | 2.48 (48.0) | 2.48 (48.0) | 0.94 (38.0) | <0.001 |
| FVC L (%) | 4.35 (78.0) | 4.38 (76.0) | 2.62 (46.0) | < 0.001 | 3.08 (54.0) | 3.08 (54.0) | 2.13 (54.0) | <0.001 |
| Post-bronchodilatador FEV1/FVCration, % | 80.29 ± 8.31 | 82.22 ± 5.55 | 46.37 ± 7.97 | < 0.001 | 83.20 ± 6.84 | 83.20 ± 6.84 | 46.85 ± 13.42 | <0.001 |
Results are expressed as frequencies (percentages) and mean ± standard deviation.
BMI: body mass index; COPD: chronic obstructive pulmonary disease; FEV1: forced expiratory volume in 1 s; FVC: forced vital capacity.
Multivariate men model for independent variables associated with COPD.
| Prevalence | 95%CI | p value | |
|---|---|---|---|
| Age (years) | 9.7% | 8.6∼10.9 | <0.0001 |
| 20−39 | 1.7% | 1.0∼2.5 | |
| 40−49 | 9.9% | 7.2∼12.6 | |
| 50−59 | 13.1% | 10.2∼16.1 | |
| 60−69 | 23.0% | 18.6∼27.5 | |
| ≥70 | 36.4% | 14.5∼58.2 | |
| Resident | 9.7% | 8.6∼10.9 | 0.422 |
| Urban residents | 9.6% | 8.4∼10,8 | |
| Rural residents | 11.4% | 6.8∼16.1 | |
| Smoking history (pack/year) | 9.4% | 8.2∼10.5 | <0.0001 |
| 0 | 17.6% | 14.9∼20.2 | |
| 1∼9 | 3.2% | 1.6∼4.8 | |
| 10∼19 | 6.3% | 3.5∼9.1 | |
| ≥20 | 6.3% | 4.7∼7.9 | |
| Smoking status | 9.7% | 8.6∼10.9 | <0.0001 |
| Never | 6.6% | 4.9∼8.4 | |
| Former smoker | 10.5% | 8.9∼12.1 | |
| Current smoker | 15.3% | 10.6∼20.0 | |
| BMI (kg/m2) | 9.7% | 8.6∼10.9 | 0.114 |
| Chronic cough during childhood (age ≤ 14 years) | 9.7% | 8.5∼10.9 | 0.009 |
| Frequent | 21.8% | 10.6∼33.1 | |
| Sometimes | 9.9% | 5.5∼14.3 | |
| Rare | 9.4% | 8.2∼10.6 | |
| History of pnumonia or brinchitis during childhood | 9.1% | 7.9∼10.3 | 0.172 |
| Yes | 12.2% | 6.9∼17.6 | |
| No | 8.9% | 7.6∼10.1 | |
| Parental history of respiratory diseases | 9.7% | 8.6∼10.9 | |
| History of spirometry test | 9.6% | 8.5∼10.8 | 1.000 |
| Yes | 9.6% | 8.5∼10.8 | |
| No | 9.1% | 11.2∼29.3 | |
| History of tuberculosis | 9.7% | 8.6∼10.9 | 0.317 |
| Yes | 14.3% | 3.2∼25.3 | |
| No | 9.7% | 8.5∼10.8 |
BMI: body mass index; CI: confidence interval.
Multivariate women model for independent variables associated with COPD.
| Prevalence | 95%CI | p value | |
|---|---|---|---|
| Age (years) | 3.9% | 3.2∼4.6 | <0.0001 |
| 20−39 | 1.2% | 0.5∼1.9 | |
| 40−49 | 3.2% | 1.9∼4.5 | |
| 50−59 | 4.6% | 3.2∼5.9 | |
| 60−69 | 8.2% | 5.7∼10.7 | |
| ≥ 70 | 5.0% | 5.5∼15.5 | |
| Resident | 3.9% | 3.2∼4.7 | 0.242 |
| Urban residents | 4.0% | 3.3∼4.7 | |
| Rural residents | 2.6% | 0.7∼4.5 | |
| Smoking history (pack/year) | 3.9% | 3.8∼5.1 | <0.0001 |
| 0 | 25.0% | 9.1∼40.9 | |
| 1∼9 | 11.1% | 0.3∼21.9 | |
| 10∼19 | 11.1% | 5.0∼27.2 | |
| ≥ 20 | 3.5% | 2.9∼4.2 | |
| Smoking status | 3.9% | 3.2∼4.6 | <0.0001 |
| Never | 3.5% | 2.9∼4.2 | |
| Former smoker | 12.5% | 4.7∼20.3 | |
| Current smoker | 20.8% | 3.3∼38.4 | |
| BMI (kg/m2) | 3.9% | 3.2∼4.6 | 0.393 |
| Chronic cough during childhood (age ≤ 14 years) | 3.9% | 3.2∼4.6 | <0.0001 |
| Frequent | 14.4% | 8.0∼20.8 | |
| Sometimes | 4.7% | 1.8∼7.5 | |
| Rare | 3.4% | 2.7∼4.1 | |
| Histoy of pnumonia or bronchitis during childhood | 3.8% | 3.1∼4.5 | <0.0001 |
| Yes | 9.9% | 5.7∼14.1 | |
| No | 3.3% | 2.6∼4.0 | |
| Parental history of respiratory diseases | 3.9% | 3.2∼4.6 | |
| History of spirometry test | 3.9% | 3.2∼4.6 | 0.197 |
| Yes | 3.9% | 3.2∼4.5 | |
| No | 9.5% | 4.2∼23.2 | |
| History of tuberculosis | 3.9% | 3.2∼4.6 | 0.553 |
| Yes | 2.6% | 1.0∼6.2 | |
| No | 3.9% | 3.2∼4.6 |
BMI: body mass index; CI: confidence interval.
More than 90% of the participants were urban residents, and the average age was 43.8 ± 14.0 for men and 46.9 ± 12.6 for women. Furthermore, compared to the less educated population (high school or lower), the prevalence of COPD was significantly lower in the higher education population (college or higher).
Smoking was also an important factor for COPD prevalence. The age-specific and age-standardized prevalence of spirometry-defined COPD was furthered calculated for the non-smoking group and the smoking group (Supplementary Table 2). The non-smoking and smoking populations showed differences in all age groups. A total of 253 subjects were current smokers (229 men and 24 women), and the prevalence of COPD calculated by age showed that the current smoking group (15.8%, 95% CI 11.3–20.3) and the former smoking group (10.6%, 95% CI 9.0–12.1) were significantly higher than the non-smoking group (4.2%, 95% CI 3.5–4.8, p < 0.0001). There was an obvious dose-response relationship between smoking years and the COPD prevalence (p < 0.0001). However, living with smokers was not related to an increase in COPD prevalence (p = 0.870).
Chronic cough in childhood (p < 0.0001) and parents with respiratory illness history (p < 0.0001) were related to an increase in COPD prevalence, and biomass utilization was also significantly associated with a rise in COPD prevalence in men (p < 0.0001).
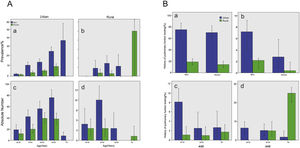
Body mass index (BMI) was 25.16 ± 3.78 kg/m2 in men and 24.61 ± 3.78 kg/m2 in women, respectively. The BMI did not show any significant effect on COPD, and there was also no difference in COPD prevalence between rural (6.1%, 95% CI 3.9–8.4) and city (6.5%, 95% CI 5.8–7.1, p = 0.785) regions (Table 2) (Fig. 2A).
COPD in population over 20 years old in Shanxi Province. A) Prevalence and absolute number of COPD in population over 20 years old in Shanxi Province. Bars represent percentage or average, and error bars represent 95% CI. The age-specific prevalence of COPD in men and women in urban regions (a) and rural regions (b). The number of COPD cases in men and women by age in urban regions (c) and in rural regions (d). B) Proportion of participants performed lung function test before or COPD cases in population over 20 years old in Shanxi Province. Bars represent proportions, and error bars represent 95% CI. Proportion with lung function test history in COPD cases, by gender and urbanization (a). Proportion with lung function test history in all participants, by gender and urbanization (b). Proportion with lung function test history in COPD cases, by age and urbanization (c). Proportion with lung function test history in all the participants, by age and urbanization (d).
Only 1‰ (5/5636) patients reported typical symptoms of COPD in their daily life, such as continuous cough and recurrent asthma. Nor were exposure to PM2.5 and tuberculosis history associated with COPD prevalence in the non-smoking and smoking groups.
Multivariable logistic regression analysis of the risk factors for COPDThe multiple-adjusted analysis showed that sex (p < 0.0001), age (p < 0.0001), and education level (p < 0.0001) were significantly related to the prevalence risk of COPD (Supplementary Table 3). The number of smoking years (p = 0.004) was positively correlated with the risk of COPD.
It should be noted that chronic cough in childhood (age ≤ 14 years, p < 0.0001) and parents with respiratory disease history (p = 0.006) were also significantly correlated with the prevalence of COPD in Shanxi. However, the other factors including rural residents, living with smokers, pneumonia or bronchitis history in childhood, BMI, biomass utilization, exposure to PM2.5, and parental tuberculosis history were not significantly correlated with COPD.
Among COPD patients identified by spirometry in our study, only five urban residents were previously aware that they had COPD disease (one man and four women). In addition, the percentage with lung examination history was higher in the city (Fig. 2B). Young COPD patients were more likely to undergo pulmonary testing.
DiscussionThis is the first time that the prevalence and risk factors of COPD in Shanxi Province have been reported based on a representative sample of adults aged 20 and above. Comparing the COPD prevalence in Shanxi Province with that of China as a whole, the COPD prevalence in Shanxi (6.4%) was lower than that in China (8.6%, 2015),8 while the proportion of COPD patients in 20−39 years old population in Shanxi (0.55%) was similar to that in China (0.46%, 2015).8
Perhaps due to the air pollution, the prevalence of COPD is becoming more and more common among young people.16 In addition, 9.3% subjects aged 40 or above were identified as COPD patients in Shanxi, which was lower than the national estimate in China (about 13.6%, 2014–2015) but higher than the former nationwide survey (8.2%, 2002–2004).7,9
Our result demonstrated that most of COPD patients could be aware their disease, which was consistent with the national survey results. For the same region and gender, the prevalence of COPD could increase with age, and males showed a higher prevalence rate than females, which was also consistent with the national data.8
The prevalence of COPD was also reported in various global regions based on cross-sectional surveys.17–19 In the BOLD study,17 9425 participants from 12 countries were used to estimate the prevalence of COPD as 10.2% overall (11.8% for men and 8.5% for women) in 2007, which was higher than our results. In the EPISCAN study18 on a population aged 40 years or older in all 17 regions of Spain, the prevalence of COPD was estimated as 11.8% (14.6% in men and 9.4% in women). The U.S. prevalence of COPD was also calculated as 10.2% in 2007–2010 using the National Health and Nutrition Examination Survey (NHANES).19 Different demographic data and exposure levels of risk factors for COPD might be the reason behind the differences in various world regions. In reported studies, the prevalence was generally higher in men than it was in women.
Smoking was considered the only main preventable factor for COPD among all risk factors in previous studies,20,21 and about 20% current smokers and 50% passive smokers were suffering from COPD in Shanxi.22,23
Considering the high COPD prevalence caused by smoking, stopping smoking should be an important strategy to reduce the number of COPD patients. Because smokers are exposed to the tar and nicotine fumes from cigarette smoke for a long time, neutrophils and macrophages are recruited and activated, releasing serine and matrix metalloproteinases, which could further activate the oxidative stress response to eliminate foreign elements. When the extracellular matrix is destroyed and cell death is beyond its repair capacity, emphysema can occur, which may contribute to COPD. In addition, passive smoking was also related to COPD in previous epidemiological research, but the number of smokers in one family did not show any relationship to COPD in our study,24,25 which may be influenced by the small sample size and different dust and chemical compositions in different regions.
The multiple-adjusted analysis demonstrated that age, sex, educational level, and chronic cough history in childhood were positively related to the risk of COPD. The incidence of COPD increased gradually with age, especially in rural regions.26–28 With an increase in age, organ structure decreases and the chest structure changes, and the airway is remodeled by stimulating chronic inflammatory factors that may lead to COPD.29
Education level is an important indicator of social economic status and health services that are closely related to the risk of disease, including COPD.30 A family history of respiratory diseases was positively correlated with the prevalence of COPD, which was consistent with previous studies.8,9 History of pneumonia or bronchitis in childhood was related to the prevalence of COPD, but the overall prevalence was diminished after using the multivariate adjustment model for comprehensive adjustment. There was no evidence that there was an association between BMI and COPD, probably because most people had a normal BMI. Compared to the non-smoking population, the biomass utilization of the whole population was less related to the prevalence of COPD, and the study by Wang et al. demonstrated that environmental pollution might cover the impact of biomass fuels.8 Apart from that, there might be a bias in the parents’ recollection of the history of tuberculosis.
Our study presented several limitations. First, female participants were oversampled because many men work outside of their area, which may lead to a decrease in COPD prevalence.
Second, the proportion of urban residents in our sample was higher than that in Shanxi Province due to the difficult of sampling in the rural area. Although our results showed no difference in the COPD prevalence between rural (6.1%, 95% CI 3.9–8.4) and city (6.5%, 95% CI 5.8–7.1, p = 0.785) regions, that may be limited by the small sample size of the rural population. In future analyses, we will try to increase the data collection on rural residents.
Third, the relationship between passive smoking and COPD risk could not be explored deeply because of a lack of detailed data about passive smoking.
Fourth, some records may present recall bias, which cross-sectional surveys cannot avoid, such as recollection of a history of tuberculosis or a previous diagnosis of COPD.
Finally, although the results of this research were comparable to the national surveys, small subgroup samples may limit the accuracy of the assessment.
ConclusionsIn conclusion, the prevalence of COPD was high in Shanxi Province, though few COPD patients performed the respiratory function tests to diagnosis it. Smoking is the main factor which could be used to prevent COPD.
Due to the high rates of underdiagnosis of COPD in Shanxi, COPD prevention and early detection should be public health priorities in Shanxi to decrease the mortality of COPD.
FundingThe Ministry of Health and Ministry of Science and Technology of China provided project research funds and reviewed the use of funds.
Conflicts of interestsThe authors declare that there are no conflicts of commercial interest related to this paper.
We would like to thank the medical staff that participated in the survey at Shanxi Bethune hospital: Liu Hu, Li Jing, Han wen, Wang Pengfei, Cao Jing, Duan Wei, Cheng Mengyu y Li Ping. We would also like to thank the graduate students of those years: Wang Junyan, Cao Yanhua, Ma Lijuan, Han Tingjiao y Zhang Tiemei; and other medical institutions: the hospital of Sinochem Second Construction Group co, Ltd, Taiyuan, Shanxi Province (China). Staff hospital of Jinxi Machinery Industry Group Co., Ltd Taiyuan, Shanxi Province (China). Nanzhai Community Health Service Center, Taiyuan, Shanxi Province (China). Xiaoyi people's Hospital, Lvliang, Shanxi Province (China). Staff hospital of JinChai Machinery Manufacturing Co., Ltd, Datong, Shanxi Province (China).
Please cite this article as: Wang R, Xu J, Wang Y. Encuesta poblacional sobre la prevalencia y los factores de riesgo de la enfermedad pulmonar obstructiva crónica en la provincia de Shanxi (China). Rev Clin Esp. 2022;222:218–228.