This work aims to assess whether symptoms/signs of congestion in patients with acute heart failure (AHF) evaluated in hospital emergency departments (HED) allows for predicting short-term progress.
Patients and methodsThe study group comprised consecutive patients diagnosed with AHF in 45 HED from EAHFE Registry. We collected clinical variables of systemic congestion (edema in the lower extremities, jugular vein distention, hepatomegaly) and pulmonary congestion (dyspnea on exertion, paroxysmal nocturnal dyspnea, orthopnea, and pulmonary crackles) and analysed their individual and group association with all-cause 30-day of mortality crudely and adjusted for differences between groups.
ResultsWe analysed 18,120 patients (median = 83 years, interquartile range [IQR] = 76–88; women = 55.7%). Of them, 44.6% had > 3 congestive symptoms/signs. Individually, the 30-day adjusted risk of death increased 14% for jugular vein distention (hazard ratio [HR] = 1.14, 95% confidence interval [95%CI] = 1.01–1.28) and 96% for dyspnea on exertion (HR = 1.96, 95% CI = 1.55–2.49). Assessed jointly, the risk progressively increased with the number of symptoms/signs present; compared to patients without symptoms/signs of congestion, the risk increased by 109%, 123 %, and 156% in patients with 1–2, 3–5, and 6–7 symptoms/signs, respectively. These associations did not show interaction with the final disposition of the patient after their emergency care (discharge/hospitalization) with the exception of edema in the lower extremities, which had a better prognosis in discharged patients (HR = 0.66, 95% CI = 0.49−0.89) than hospitalised patients (HR = 1.01, 95% CI = 0.65–1.57; interaction p < 0.001).
ConclusionThe presence of a greater number of congestive symptoms/signs was associated with greater all-cause 30-day mortality. Individually, jugular vein distention and dyspnea on exertion were associated with higher short-term mortality.
: Evaluar si los síntomas/signos de congestión en pacientes con insuficiencia cardíaca aguda (ICA) atendidos en los servicios de urgencias hospitalarios (SUH) permiten predecir la evolución a corto plazo.
Pacientes y métodosPacientes consecutivos diagnosticados de ICA en 45 SUH del Registro EAHFE. Recogimos variables clínicas de congestión sistémica (edemas en miembros inferiores, ingurgitación yugular, hepatomegalia) y pulmonar (disnea de esfuerzo, disnea paroxística nocturna, ortopnea y crepitantes pulmonares) analizando su asociación con la mortalidad por cualquier causa a 30 días, de forma cruda y ajustada por diferencias entre grupos.
ResultadosAnalizamos 18.120 pacientes (mediana = 83 años, rango intercuartil [RIC] = 76–88; mujeres = 55,7%). El 44,6% presentaba >3 síntomas/signos congestivos. Individualmente, el riesgo ajustado de muerte a 30 días se incrementó un 14% para la existencia de ingurgitación yugular (hazard ratio [HR] = 1,14, intervalo de confianza al 95% [IC95%] = 1,01−1,28) y un 96% para la disnea de esfuerzo (HR = 1,96, IC95% = 1,55−2,49). Valorados conjuntamente, el riesgo se incrementó progresivamente con el número de síntomas/signos presentes; así, respecto a los pacientes sin síntomas/signos de congestión, el riesgo incrementó un 109%, 123% y 156% en pacientes con 1–2, 3–5 y 6–7 síntomas/signos respectivamente. Estas asociaciones no mostraron interacción con la disposición final del paciente tras su atención en Urgencias (alta/hospitalización), con excepción de edemas en extremidades inferiores, que tuvieron mejor pronóstico en pacientes dados de alta (HR = 0,66, IC95% = 0,49−0,89) que en los hospitalizados (HR = 1,01, IC95% = 0,65−1,57; p interacción <0,001).
ConclusiónLa presencia de mayor número de síntomas/signos congestivos se asoció a una mayor mortalidad de cualquier causa a los 30 días. Individualmente, la ingurgitación yugular y la disnea de esfuerzo se asocian a mayor mortalidad a corto plazo.
Acute heart failure (AHF) is the onset or worsening of signs and symptoms of heart failure (HF) that require emergency treatment,1,2 and is one of the main causes of morbidity and mortality around the world. Despite the variation among clinical profiles and the heterogeneity of the underlying causes, the majority of patients with AHF present signs/symptoms of pulmonary and systemic congestion rather than low cardiac output.
Multiple studies, conducted in both admitted patients with AHF3 and in hospital emergency departments (HED), show that congestion is present in over 90% of cases, regardless of the left ventricular ejection fraction.4
Signs/symptoms of congestion are easily assessed at point-of-care by the physician and require optimal medical history and thorough physical examination. Currently, the considerable number of supplementary exams that make it possible to establish a clinical diagnosis (radiology, labs, ultrasound) seem to have displaced the importance of this clinical semiology.5,6 However, these clinical parameters offer the advantage of their easy procurement. What’s more, it is possible that some of these signs/symptoms of congestion could be associated with poor progress, though this has not been specifically evaluated in patients attended to in HEDs.
Therefore, the main objective of this study was to determine if the clinical parameters of pulmonary and systemic congestion, analysed individually or jointly, can predict early mortality in patients with AHF cared for in the HED.
MethodStudy scopeSecondary analysis of the registry Epidemiology of Acute Heart Failure in Emergency departments (EAHFE). Multicentre, multi-purpose, non interventional, analytical registry with prospective follow-up including consecutive patients who attended the HED for an episode of AHF. Six (6) recruitment phases were conducted (in 2007, 2009, 2011, 2014, 2016, and 2018) in a total of 45 Spanish HEDs. The details and characteristics of these patients had already been published previously.7–10
Study designWe included all the patients from the EAHFE registry for which the following 3 clinical variables of systemic congestion had been recorded: oedema of the lower extremities (affecting both soft limbs, surpassing isolated malleolar oedema), jugular venous distention (greater than 4 cm with the patient sitting at 45°) and hepatomegaly (liver palpation that surpasses the costal margin with increased jugular repletion following compression), and the following 4 clinical variables for pulmonary congestion: dyspnoea on exertion (at least moderate exertion or greater), orthopnoea (worsening dyspnoea in decubitus), paroxysmal nocturnal dyspnoea (onset of rapidly progressive dyspnoea after a certain period in decubitus) and pulmonary crackles (bilateral and in at least both lower thirds).
These variables were classified and analysed both individually and jointly (in this case, the patient could present 0–7 signs/symptoms of congestion).
Independent and outcome variablesWe collected a total of 20 independent variables: 2 demographic (age, sex), 9 comorbidities (arterial hypertension, diabetes mellitus, ischaemic heart disease, chronic kidney disease, cerebrovascular disease, atrial fibrillation, valvular heart disease, peripheral artery disease, chronic obstructive pulmonary disease), 4 chronic treatment variables (diuretics, angiotensin-converting enzyme inhibitors, beta blockers, aldosterone receptor antagonists), and 5 emergency management variables (treatment with intravenous diuretics, intravenous nitroglycerin, inotropic agents/vasopressors, non-invasive mechanical ventilation, need for hospital admission). The definitions of these variables are presented in the supplementary table (Appendix B supplementary data).
The primary outcome variable was 30-day all-cause mortality following the index event, which was considered the day the patient visited the emergency department. The assignment of the primary event was conducted locally by the principal investigator via telephone contact or access to the medical history.
Statistical analysisThe categorical variables were described using frequencies and percentages; the continuous variables using medians and interquartile range (IQR). The distribution analysis of the categorical variables was performed using the chi-squared test or Fisher’s exact test, as appropriate, and the Mann-Whitney test for the continuous variables.
The magnitude of the association between a specific sign/symptom of congestion and 30-day mortality was expressed as the proportional risk (hazard ratio [HR]) with its 95% confidence interval (95% CI), calculated using the Cox regression model. Initially, this was done in a univariate manner and, subsequently, 4 multivariate-adjusted models were created: model 1 was adjusted by introducing the rest of the signs and symptoms of congestion as covariates; model 2 was adjusted by the same covariates as model 1 plus age and sex; model 3 was adjusted by the same covariates as model 2 plus comorbidity (arterial hypertension, diabetes mellitus, ischaemic heart disease, chronic kidney disease, cerebrovascular disease, atrial fibrillation, peripheral artery disease, chronic obstructive pulmonary disease), and baseline treatment (diuretics, renin-angiotensin inhibitors, beta blockers, and mineralocorticoid receptor antagonists). Model 4 was adjusted by the same covariates as model 3 plus management during the decompensation episode (diuretics, intravenous vasodilators and inotropic agents/vasopressors, non-invasive mechanical ventilation; model with total adjustment). In the model with total adjustment, the existence of an interaction between sign/symptom and the fact that the patient was hospitalised or directly discharged from the ED was analysed.
All the mortality analyses mentioned were repeated, forming 4 groups according to the number of signs/symptoms the patient presented (0, 1–2, 3–5, and 6–7) and the group without congestion (0) was taken as the reference group.
In all the comparisons, it was accepted that the differences were statistically significant if the p-value was less than 0.05, or if the 95% CI for HR excluded the number 1. The statistical analysis was performed with SPSS® program version 24.0 for Windows (IBM Corp. Released 2016. IBM SPSS Statistics for Windows, Version 24.0. Armonk, NY: IBM Corp).
Ethical aspectsThe ethical principles of the Declaration of Helsinki on medical research involving humans were followed and all the patients were asked to sign an informed consent in order to participate in the study.
The protocol was approved by the Hospital Universitario Central de Asturias’ Research Ethics Committee as the main committee (protocols 49/2010, 69/2011, 166/13, 160/15, and 205/17) and by the rest of the participating centres.
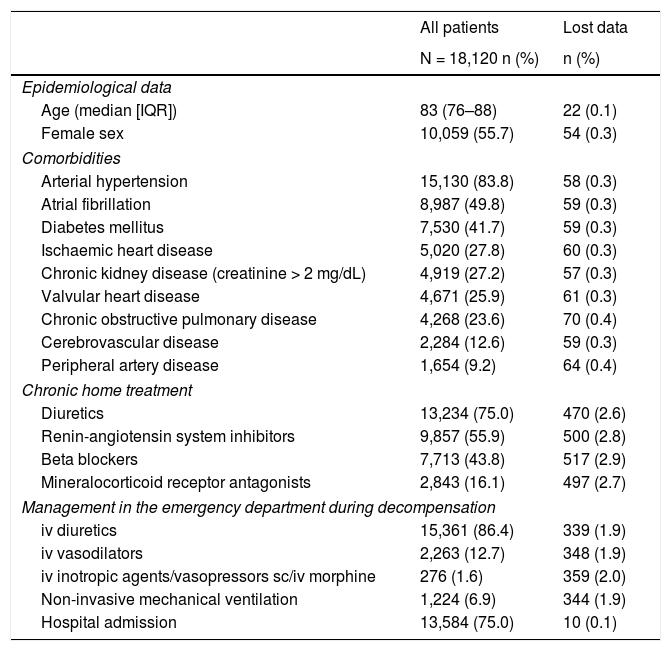
ResultsOf the 18,370 patients included in the EAHFE registry, the study population included 18,120 (98.6%) for which signs/symptoms of congestion were collected, this being the subject of research. More than half were female with a mean age of 83 (IQR = 76–88). The rest of the clinical characteristics of the study patients are presented in Table 1.
Characteristics of the 18,120 patients included in this study.
| All patients | Lost data | |
|---|---|---|
| N = 18,120 n (%) | n (%) | |
| Epidemiological data | ||
| Age (median [IQR]) | 83 (76–88) | 22 (0.1) |
| Female sex | 10,059 (55.7) | 54 (0.3) |
| Comorbidities | ||
| Arterial hypertension | 15,130 (83.8) | 58 (0.3) |
| Atrial fibrillation | 8,987 (49.8) | 59 (0.3) |
| Diabetes mellitus | 7,530 (41.7) | 59 (0.3) |
| Ischaemic heart disease | 5,020 (27.8) | 60 (0.3) |
| Chronic kidney disease (creatinine > 2 mg/dL) | 4,919 (27.2) | 57 (0.3) |
| Valvular heart disease | 4,671 (25.9) | 61 (0.3) |
| Chronic obstructive pulmonary disease | 4,268 (23.6) | 70 (0.4) |
| Cerebrovascular disease | 2,284 (12.6) | 59 (0.3) |
| Peripheral artery disease | 1,654 (9.2) | 64 (0.4) |
| Chronic home treatment | ||
| Diuretics | 13,234 (75.0) | 470 (2.6) |
| Renin-angiotensin system inhibitors | 9,857 (55.9) | 500 (2.8) |
| Beta blockers | 7,713 (43.8) | 517 (2.9) |
| Mineralocorticoid receptor antagonists | 2,843 (16.1) | 497 (2.7) |
| Management in the emergency department during decompensation | ||
| iv diuretics | 15,361 (86.4) | 339 (1.9) |
| iv vasodilators | 2,263 (12.7) | 348 (1.9) |
| iv inotropic agents/vasopressors sc/iv morphine | 276 (1.6) | 359 (2.0) |
| Non-invasive mechanical ventilation | 1,224 (6.9) | 344 (1.9) |
| Hospital admission | 13,584 (75.0) | 10 (0.1) |
IQR: interquartile range; iv: intravenous; sc: subcutaneous.
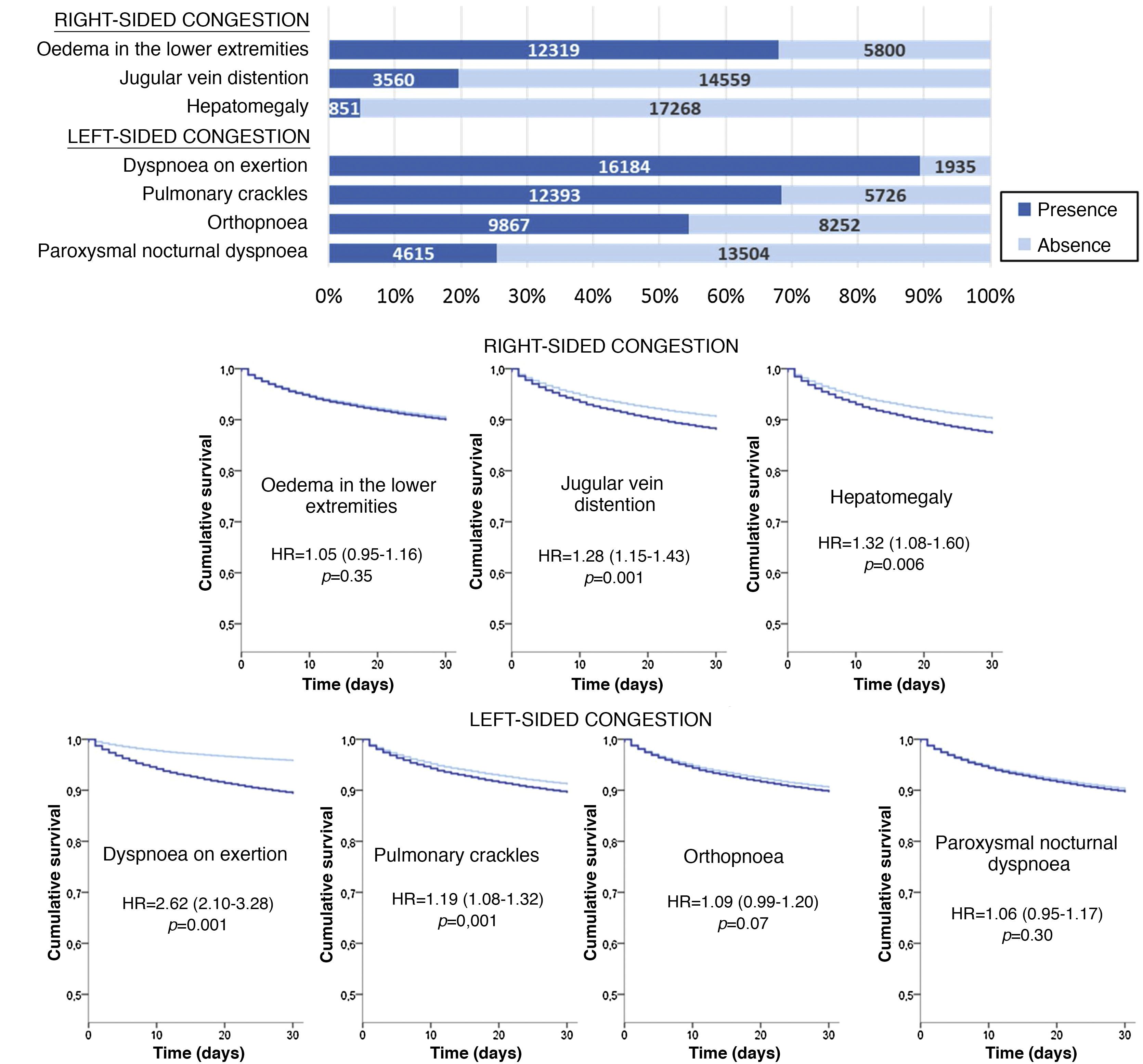
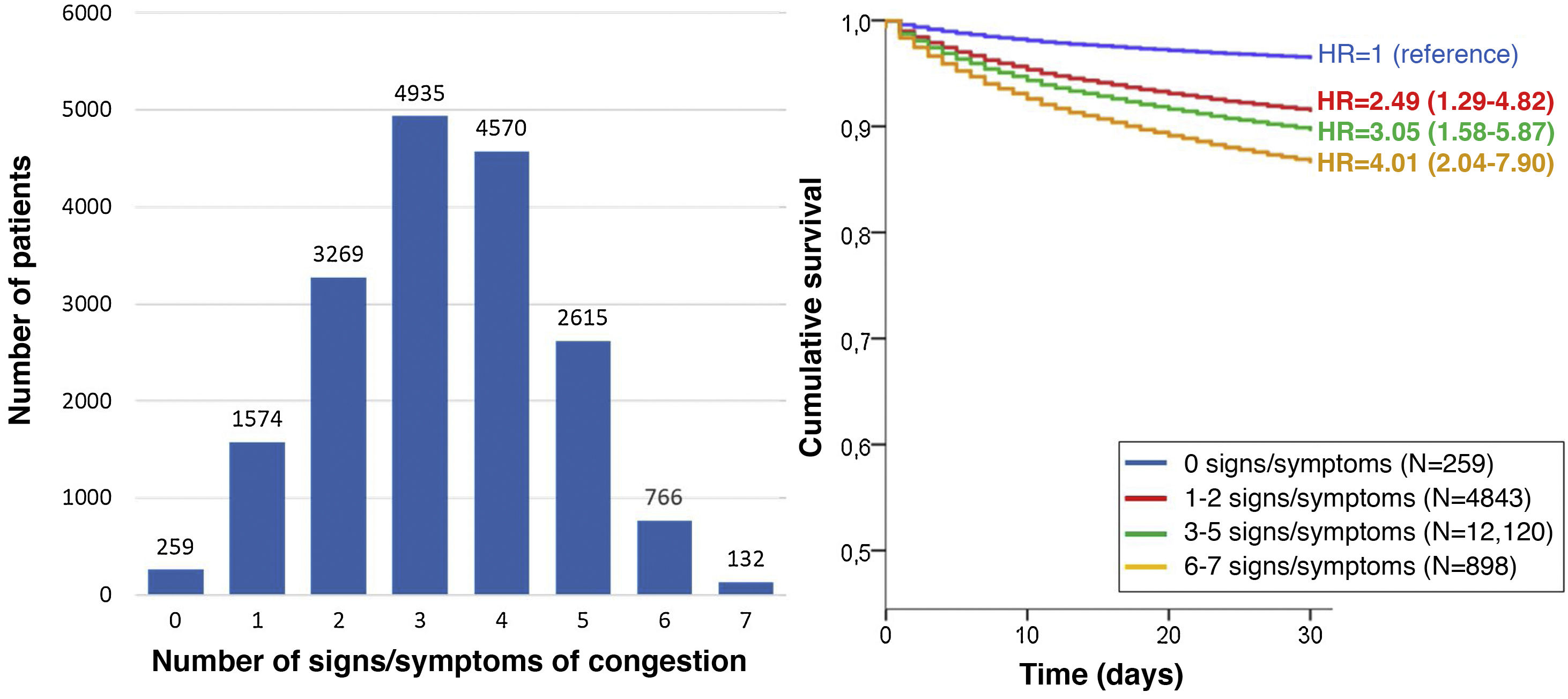
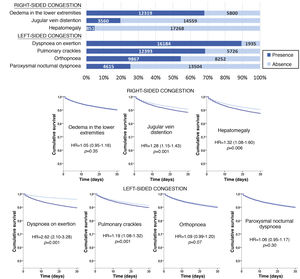
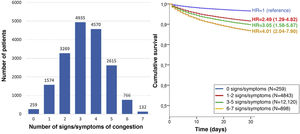
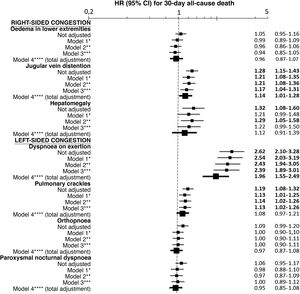
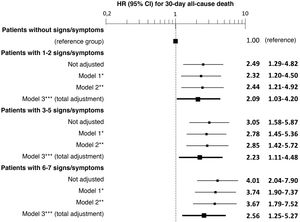
The most frequent sign/symptom of systemic congestion, observed in 12,319 patients, was oedema of the lower limbs, and the most frequent for pulmonary congestion was dyspnoea on exertion, present in 16,184 patients (Fig. 1). A total of 98.6% presented at least one sign/symptom of congestion, and 44.6% presented 4 or more (Fig. 2).
Follow-up was possible for 18,119 patients (one patient lost to follow-up) and 1781 patients died in the 30 days after receiving care at the HED. Cumulative mortality for the series was 9.9% (95% CI = 9.5–10.3). In the univariate analysis, a statistically significant association was observed between 30-day mortality and the presence of jugular venous distention, hepatomegaly, dyspnoea on exertion, and pulmonary crackles, but not with oedema of the lower extremities, orthopnoea, or paroxysmal nocturnal dyspnoea (Fig. 1).
In addition, a progressive increase in the risk of 30-day mortality was observed as the number of signs/symptoms of congestion increased, reaching a HR of 4.01 (95% CI = 2.04–7.90) in the patients with 6–7 signs/symptoms (Fig. 2). Regarding the patients without clinical signs and symptoms of congestion, 30-day mortality was higher in the patients showing exclusively systemic congestion symptoms (HR = 2.40; 95% CI = 1.09–5.27), and was also higher in patients with exclusively left-sided congestion (HR = 2.77; 95% CI = 1.43–5.36) or in those with congestion symptoms in both territories (HR = 3.00; 95% CI = 1.56–5.77).
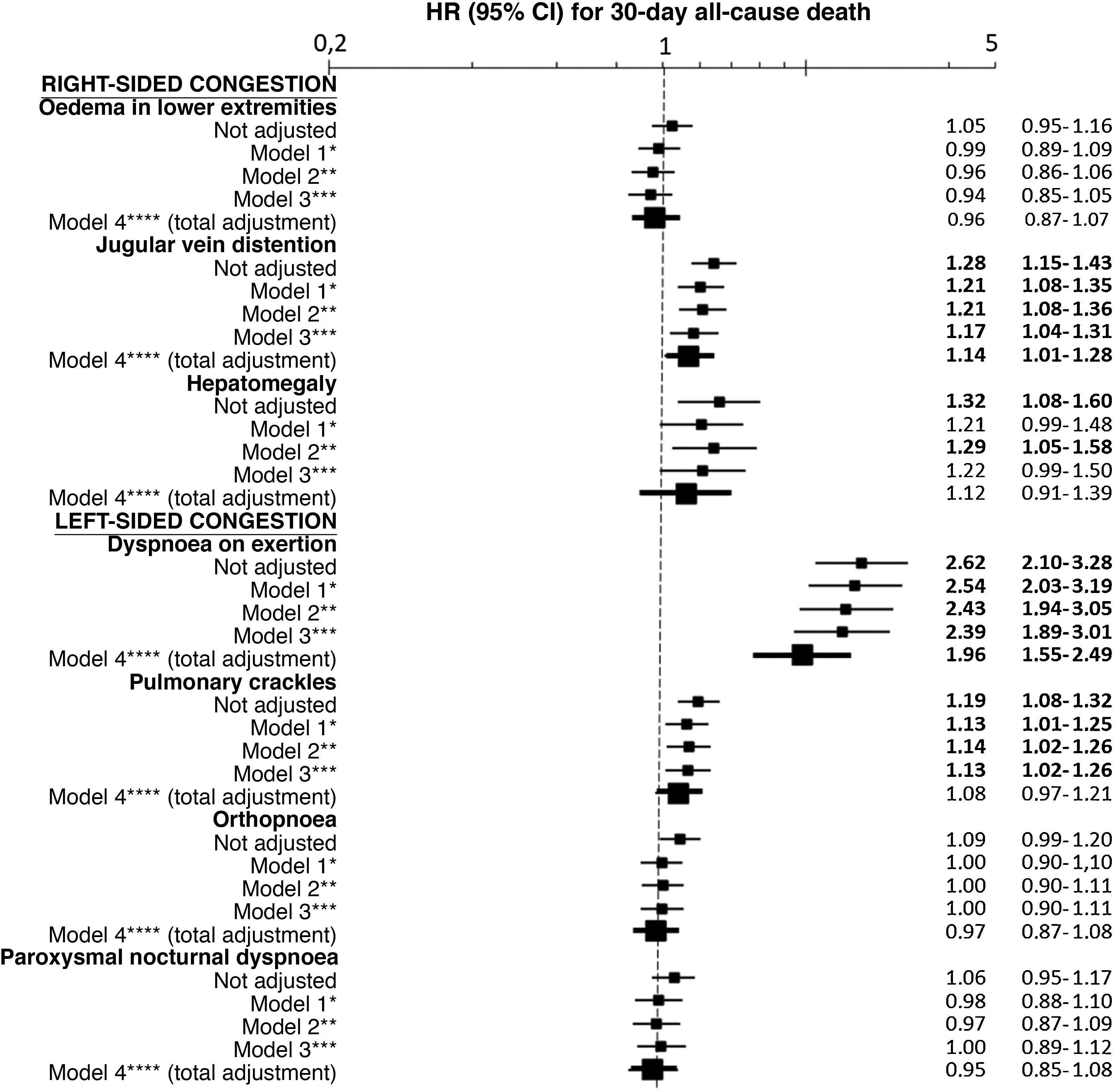
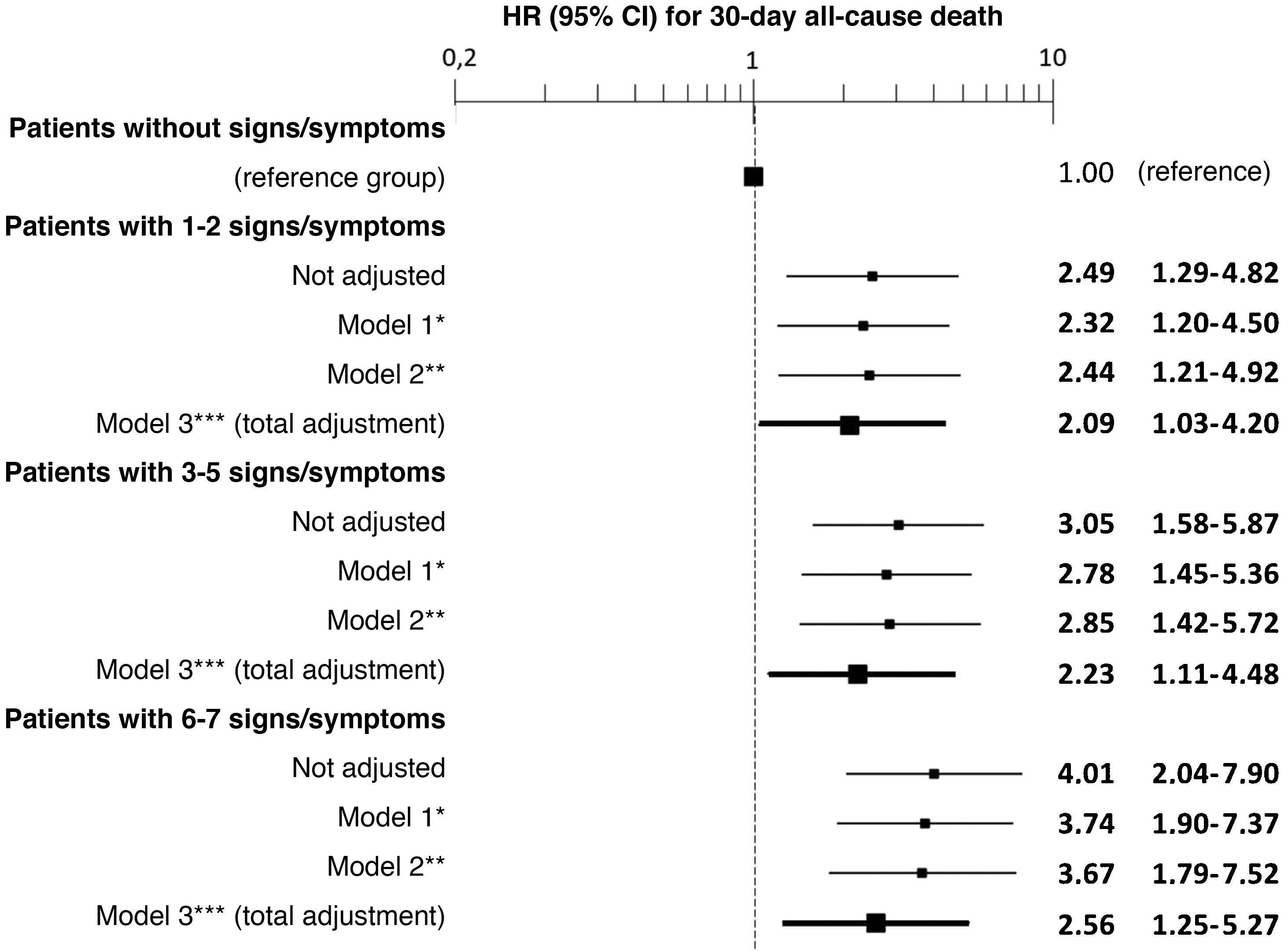
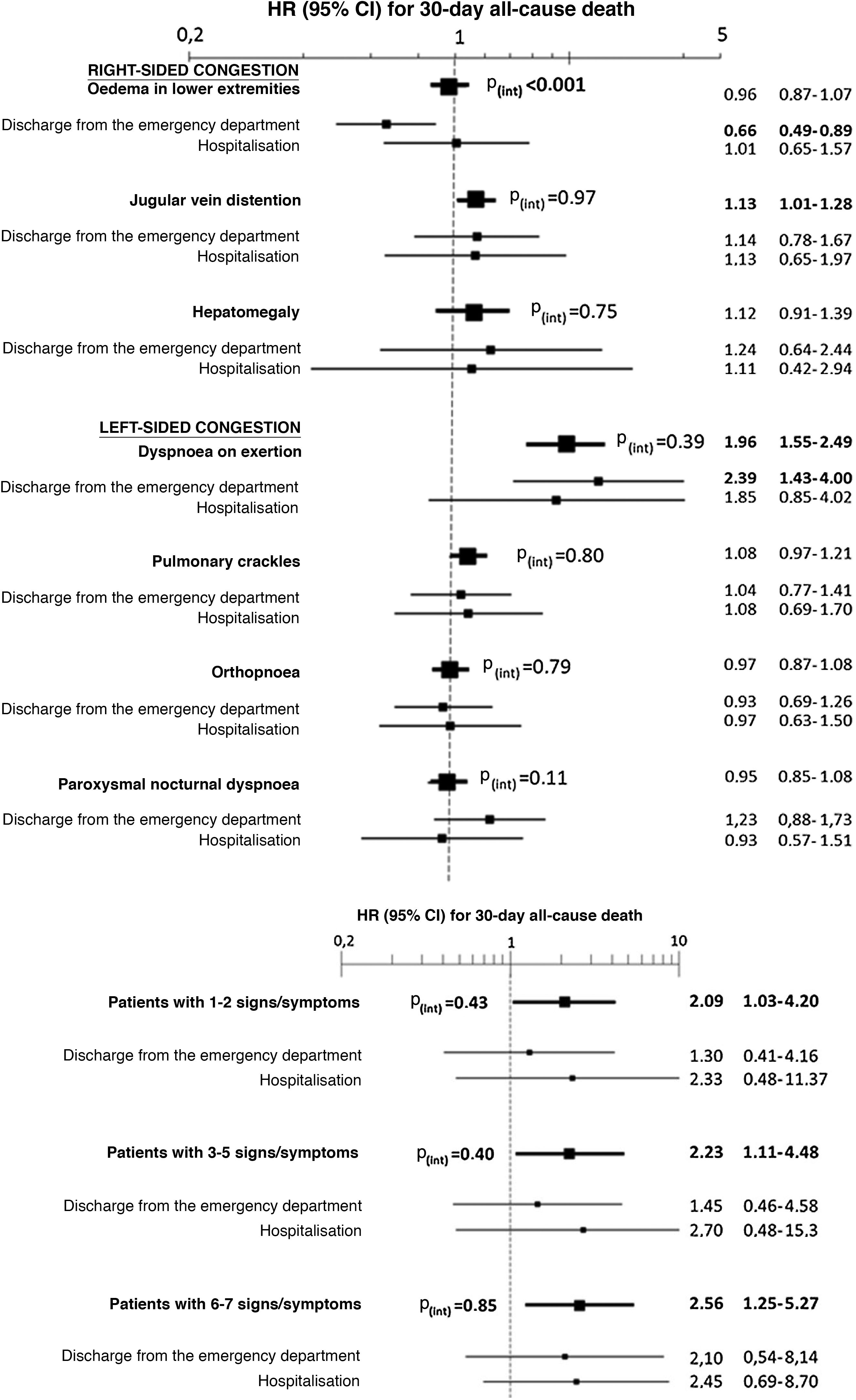
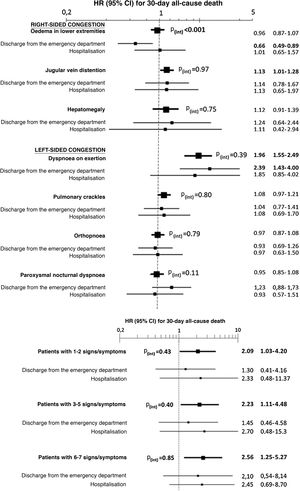
The multivariate analysis showed consistent risks in the different models, similar to those of the non-adjusted analysis, though the trend was a decrease in the magnitude of the associations (Fig. 3 and 4). In the total adjustment model, the presence of jugular venous distention increased the risk of 30-day all-cause death by 14% (HR = 1.14; 95% CI = 1.01–1.28) and dyspnoea on exertion by 96% (HR = 1.96; 95% CI = 1.55–2.49) (Fig. 3). Compared to the absence of congestion, the presence of 1–2 signs/symptoms increased risk by 109%; 3–5 simultaneous signs/symptoms by 123%, and 6–7 by 156% (Fig. 4). The analysis of the interaction between the presence of congestion and the final status of the patient after receiving emergency care did not show any significant interaction, with the exception of oedema in the lower extremities, which was associated with a better prognosis in patients discharged from the ED (HR = 0.66, 95% CI = 0.49−0.89) than in hospitalised patients (HR = 1.01, 95% CI = 0.65–1.57; interaction p < 0.001) (Fig. 5).
Multivariate-adjusted models for the association between individually evaluated different signs and symptoms of congestion and 30-day all-cause mortality.
The hazard ratios (HR) in bold indicate statistical significance (p < 0.05).
*Model 1 was adjusted by introducing the rest of signs and symptoms of congestion as covariates.
**Model 2 was adjusted by the same covariates as Model 1 plus age and sex.
***Model 3 was adjusted by the same covariates as Model 2 plus comorbidity (arterial hypertension, diabetes mellitus, ischaemic heart disease, chronic kidney disease, cerebrovascular disease, atrial fibrillation, peripheral artery disease, chronic obstructive pulmonary disease), and baseline treatment (diuretics, renin-angiotensin inhibitors, beta blockers, and mineralocorticoid receptor antagonists).
****Model 4 was adjusted by the same covariates as Model 3 plus management during the decompensation episode (diuretics, intravenous vasodilators and inotropic agents/vasopressors, non-invasive mechanical ventilation and hospitalisation).
Multivariate-adjusted models for the association between the number of signs and symptoms of congestion the patient presents and 30-day all-cause mortality.
The hazard ratios (HR) in bold indicate statistical significance (p < 0.05).
*Model 1 was adjusted by introducing age and sex as covariates.
**Model 2 was adjusted by the same covariates as Model 1 plus comorbidity (arterial hypertension, diabetes mellitus, ischaemic heart disease, chronic kidney disease, cerebrovascular disease, atrial fibrillation, peripheral artery disease, chronic obstructive pulmonary disease), and baseline treatment (diuretics, renin-angiotensin inhibitors, beta blockers, and mineralocorticoid receptor antagonists).
***Model 3 was adjusted by the same covariates as Model 2 plus management during the decompensation episode (diuretics, intravenous vasodilators and inotropic agents/vasopressors, non-invasive mechanical ventilation and hospitalisation).
Evaluation in the multivariate model with total adjustment* evaluating 30-day all-cause mortality of the interaction between the signs and symptoms of congestion taken individually (above) or grouped (below) and the patient’s status after receiving care in the emergency department (discharge/admission).
The hazard ratios (HR) in bold indicate statistical significance (p < 0.05).
*The total adjustment was performed by adding as covariates the rest of the signs and symptoms of congestion (in the case of the analysis of individual signs and symptoms—above—, not in the grouped analysis—below—), age and sex, comorbidity (arterial hypertension, diabetes mellitus, ischaemic heart disease, chronic kidney disease, cerebrovascular disease, atrial fibrillation, peripheral artery disease, chronic obstructive pulmonary disease), baseline treatment (diuretics, renin-angiotensin inhibitors, beta blockers, and mineralocorticoid receptor antagonists), and management during the decompensation episode (diuretics, intravenous vasodilators and inotropic agents/vasopressors, non-invasive mechanical ventilation, and hospitalisation).
There are three main findings in this study. The first being that signs of congestion are very frequent at the time of consultation of patients with AHF and their absence quite exceptional, this being observed in only 1% of the patients seen in the ED. The second is that, individually, some signs are associated with short-term mortality, and as the number of signs increases, so does the risk of dying. And third, the association between the presence of signs/symptoms of congestion with the mortality observed in patients discharged from the emergency department is similar to that demonstrated if the patient is hospitalised. The exception is oedema in the lower extremities, whose presence in discharged patients is associated with a better prognosis for emergency department discharge than for hospitalisation.
All these findings were obtained by analysing a cohort of over 18,000 patients diagnosed with AHF in the emergency department who globally presented very old age (mean age 83), high comorbidity, and whose 30-day mortality (from 9.9%) was within the range reported in other similar series.3,11,12
In patients with AHF in the HEDs, both pulmonary and systemic signs/symptoms of congestion tend to be most common. In the European registry ESC‐EORP‐HFA (Heart Failure Long‐Term Registry), which included 7865 patients with AHF, 89.7% had clinical signs of congestion at admission.3 Similar results have been reported in Spain, with 11,261 patients with AHF, wherein 93.1% presented signs/symptoms of congestion when seen in the HEDs.4
In this study, up to 98.6% of patients presented with one sign/symptom of congestion in the emergency department and 89.9% showed more than one sign/symptom. In the aforementioned studies, it is possible that a specific search of the complete semiology of congestion in the patients with AHF was not performed in the moment in the emergency department and that those patients could include some paucisymptomatic cases with isolated symptoms that went unnoticed.
Very few studies correlate the number of signs/symptoms with patient prognosis.13–17 In our study, the presence of a greater number of symptoms/signs was associated with greater 30-day all-cause mortality. In a recent study that included 24,724 patients from the OPTIMIZE-HF registry, which applied a congestion scale using various signs/symptoms (dyspnoea, orthopnoea, tendency to tire, jugular venous pressure [JVP], crackles, and oedemas), the patients were divided into 3 groups (low, moderate, or high grade) and it was shown that the group with a higher number of signs/symptoms had higher post-discharge mortality (at 90−180–365 days, respectively) than the other two groups.18
Individually, an association was observed in our study between both jugular distention and dyspnoea on exertion and 30-day all-cause mortality. A study in 2569 patients with HF evaluated whether JVP was an independent prognostic factor and concluded that it was associated with a higher risk of death and hospitalisation due to HF (RR = 1.30; 95% CI = 1.11–1.53; p < 0.005).13 While measurement may occasionally be limited due to various factors (obesity or pulmonary diseases), the presence of jugular venous distention is posited as one of the most useful signs for establishing prognosis in AHF.15,17 As we expected, dyspnoea on exertion is the most common persistent symptom at hospital discharge and alleviating or improving this symptom affects prognosis as it translates into the degree of resolution of congestion.19,20
It has also been shown that functional capacity on its own is predictive of mortality and the hospitalisations of patients with HF.21 This result may suggest that dyspnoea on exertion translates to HF that is likely more chronic and associated with worse prognosis. Absence of dyspnoea on exertion was very uncommon and could translate into cases of AHF with cause.2 In any case, we believe that the strong association between the “decline in dyspnoea” symptom and 30-day mortality highlights the importance of patient perception regarding the severity of the episode, which the patient fundamentally associates with worsening of their functional class. The fact that this is a symptom (though subjective) greatly underscores the importance of the medical history and giving proper consideration to the patient’s experience.
We did not find differences between the different signs/symptoms of congestion and mortality among the patients discharged directly from the emergency department versus hospitalised patients, except for the presence of oedemas in discharged patients, which was associated with better prognosis. Therefore, together with other variables, appropriate decisions can be made regarding which patients can be directly discharged. Up to 40% of patients are discharged with signs/symptoms of congestion, which has been associated with a high rate of post-discharge adverse events.22 This group represents a different clinical profile with minimal residual congestion at discharge, normal or elevated blood pressure, low rates of general or cardiac comorbidity, and non-advanced forms and better functional class.23–26
LimitationsThe signs/symptoms assessed in this study for measuring congestion can occasionally be secondary to non-cardiac diseases and may have low sensitivity for measuring congestion. Therefore, diagnosis for inclusion in the EAHFE registry is based on clinical criteria, though diagnosis is confirmed via natriuretic peptide test and/or echocardiography in close to 90% of cases. Second, the determination of each sign/symptom was conducted by a single researcher without confirmation from the independent central adjudication committee and was subject to interobserver variability (JVP in particular). The third limitation is that no clinical perfusion parameters were determined, which has been related to higher mortality. Fourth, though selection bias may have been present as we only included patients for whom the signs/symptoms subject of this study were collected, we believe said bias to be low, since these data were only missing for 1.4% of the EAHFE cohort. Lastly, congestion status at discharge following response to depletion therapy was not recorded. While the congestive status of patients discharged directly from the emergency department could not have varied greatly, we would expect changes in the status of the hospitalised patients.
ConclusionClinical congestion parameters measured at any level of care in patients with AHF, when analysed jointly, can predict the progress of AHF. The presence of a higher number of signs/symptoms of congestion was associated with an increase in short-term mortality. Jugular venous distention and dyspnoea on exertion were those associated with higher mortality. Taking into consideration the presence of these symptoms could improve patient management and prognosis and help guide individual treatment.
FundingThis study was made possible in part thanks to the Carlos III Health Institute with funding from the Ministry of Health and FEDER (PI15/01019, PI18/00393), La Marató from TV3 (2015/2510) and the Government of Catalonia for consolidated research groups (GRC 2009/1385, 2014/0313, 2017/1424).
Conflicts of interestThe authors declare that they do not have any conflicts of interest.
Marta Fuentes, Cristina Gil (H.U. de Salamanca); Héctor Alonso, Enrique Pérez-Llantada (H. Marqués de Valdecilla de Santander); Francisco Javier Martín-Sánchez, Guillermo Llopis García, Mar Suárez Cadenas (H. Clínico San Carlos de Madrid); Òscar Miró, Víctor Gil, Rosa Escoda, Sira Aguiló, Carolina Sánchez, Ana García-Álvarez (H. Clínic de Barcelona); Javier Millán (H.U. i Politécnic La Fe de Valencia); José Pavón (H. Dr. Negrín de Las Palmas de Gran Canaria); Antonio Noval (H. Insular de Las Palmas de Gran Canaria); María Luisa López-Grima, Amparo Valero, María Ángeles Juan (H. Dr. Peset de Valencia); Alfons Aguirre, María Angels Pedragosa, Silvia Mínguez Masó (H. del Mar de Barcelona); María Isabel Alonso, Francisco Ruiz (H. de Valme de Sevilla); José Miguel Franco (H. Miguel Servet de Zaragoza); Ana Belén Mecina (H. de Alcorcón de Madrid); Josep Tost, Marta Berenguer, Ruxandra Donea (Consorci Sanitari de Terrassa); Susana Sánchez Ramón, Virginia Carbajosa Rodríguez (H.U. Río Hortega de Valladolid); Pascual Piñera, José Andrés Sánchez Nicolás (H. Reina Sofía de Murcia); Raquel Torres Garate (H. Severo Ochoa de Madrid); Aitor Alquézar-Arbé, Miguel Alberto Rizzi, Sergio Herrera (H. de la Santa Creu y Sant Pau de Barcelona); Javier Jacob, Alex Roset, Irene Cabello, Antonio Haro (H.U. de Bellvitge de Barcelona); Fernando Richard, José María Álvarez Pérez, María Pilar López Diez (H.U. de Burgos); Pablo Herrero Puente, Joaquín Vázquez Álvarez, Belén Prieto García, María García García, Marta Sánchez González (H.U. Central de Asturias de Oviedo); Pere Llorens, Patricia Javaloyes, Inmaculada Jiménez, Néstor Hernández, Begoña Espinosa, Adriana Gil, Francisca Molina, Tamara García (H. General de Alicante); Juan Antonio Andueza (Hospital General Universitario Gregorio Marañón de Madrid); Rodolfo Romero (H.U. de Getafe de Madrid); Martín Ruíz, Roberto Calvache (H. de Henares de Madrid); María Teresa Lorca Serralta, Luis Ernesto Calderón Jave (H. del Tajo de Madrid); Beatriz Amores Arriaga, Beatriz Sierra Bergua (H. Clínico Lozano Blesa de Zaragoza); Enrique Martín Mojarro, Brigitte Silvana Alarcón Jiménez (H. Sant Pau i Santa Tecla de Tarragona); Lisette Travería Bécquer, Guillermo Burillo (H.U. de Canarias de Tenerife); Lluís Llauger García, Gerard Corominas LaSalle (H.U. de Vic, Barcelona); Carmen Agüera Urbano, Ana Belén García Soto, Elisa Delgado Padial (H. Costa del Sol de Marbella, Málaga); Ester Soy Ferrer, María Adroher Múñoz (H. Josep Trueta de Girona); José Manuel Garrido (H. Virgen Macarena de Sevilla); Francisco Javier Lucas-Imbernón (H. General Universitario de Albacete); Rut Gaya (H. Juan XXIII de Tarragona); Carlos Bibiano, María Mir, Beatriz Rodríguez (H. Infanta Leonor de Madrid); José Luis Carballo (Complejo Hospitalario Universitario de Ourense); Esther Rodríguez-Adrada, Belén Rodríguez Miranda, Monika Vicente Martín (H. Rey Juan Carlos de Móstoles, Madrid) y Pere Coma Casanova, Joan Espinach Alvarós (H. San Joan de Deu de Martorell, Barcelona).
The names of the components of the ICA-SEMES group are listed in Appendix A.
Please cite this article as: Espinosa B, Llorens P, Gil V, Rossello X, Jacob J, Herrero P, et al., Pronóstico de la insuficiencia cardíaca aguda basado en datos clínicos de congestión. Rev Clín Esp. 2022;222:321–331.