There is controversy regarding the best predictors of clinical deterioration in COVID-19.
ObjectiveThis work aims to identify predictors of risk factors for deterioration in patients hospitalized due to COVID-19.
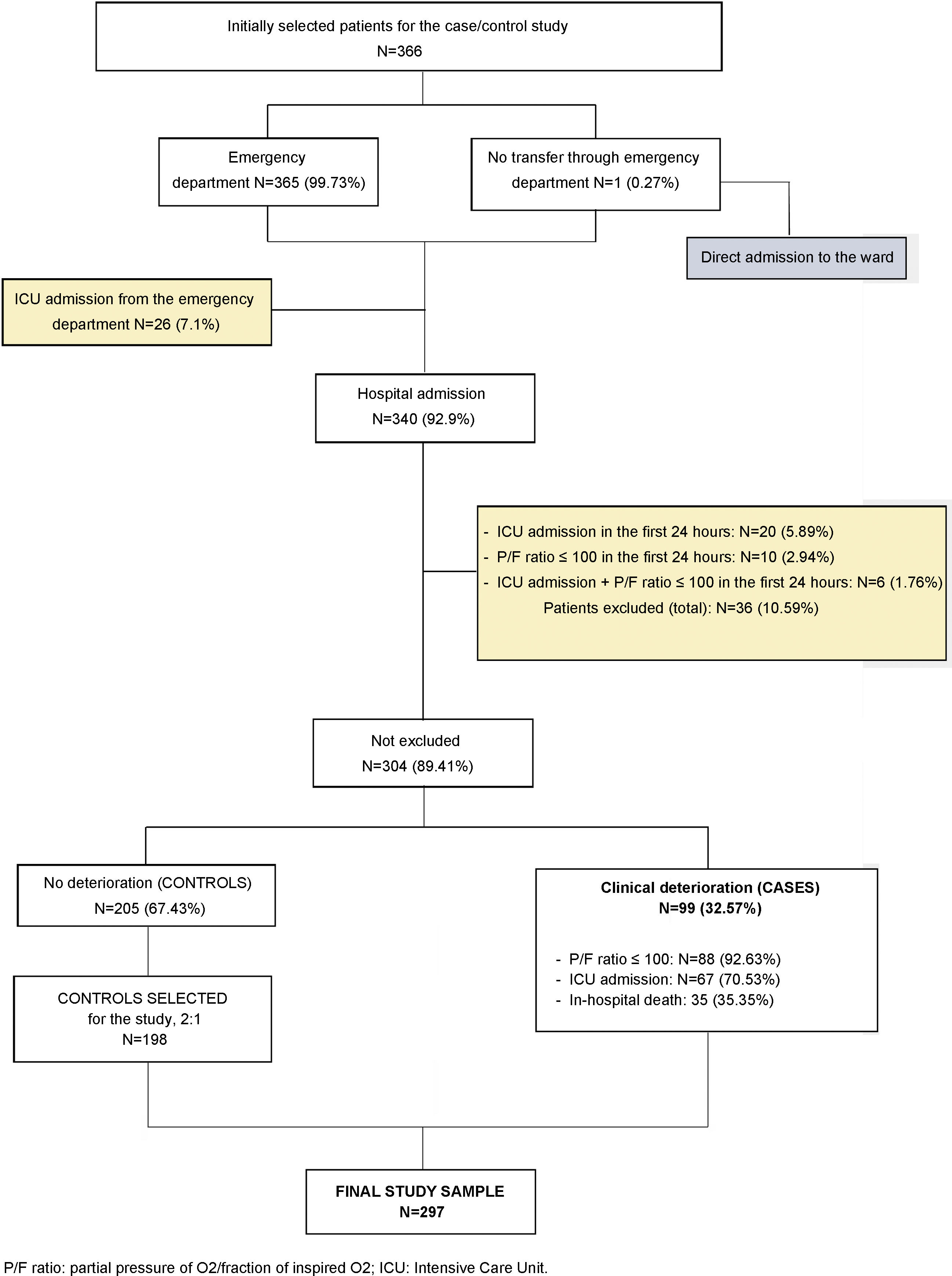
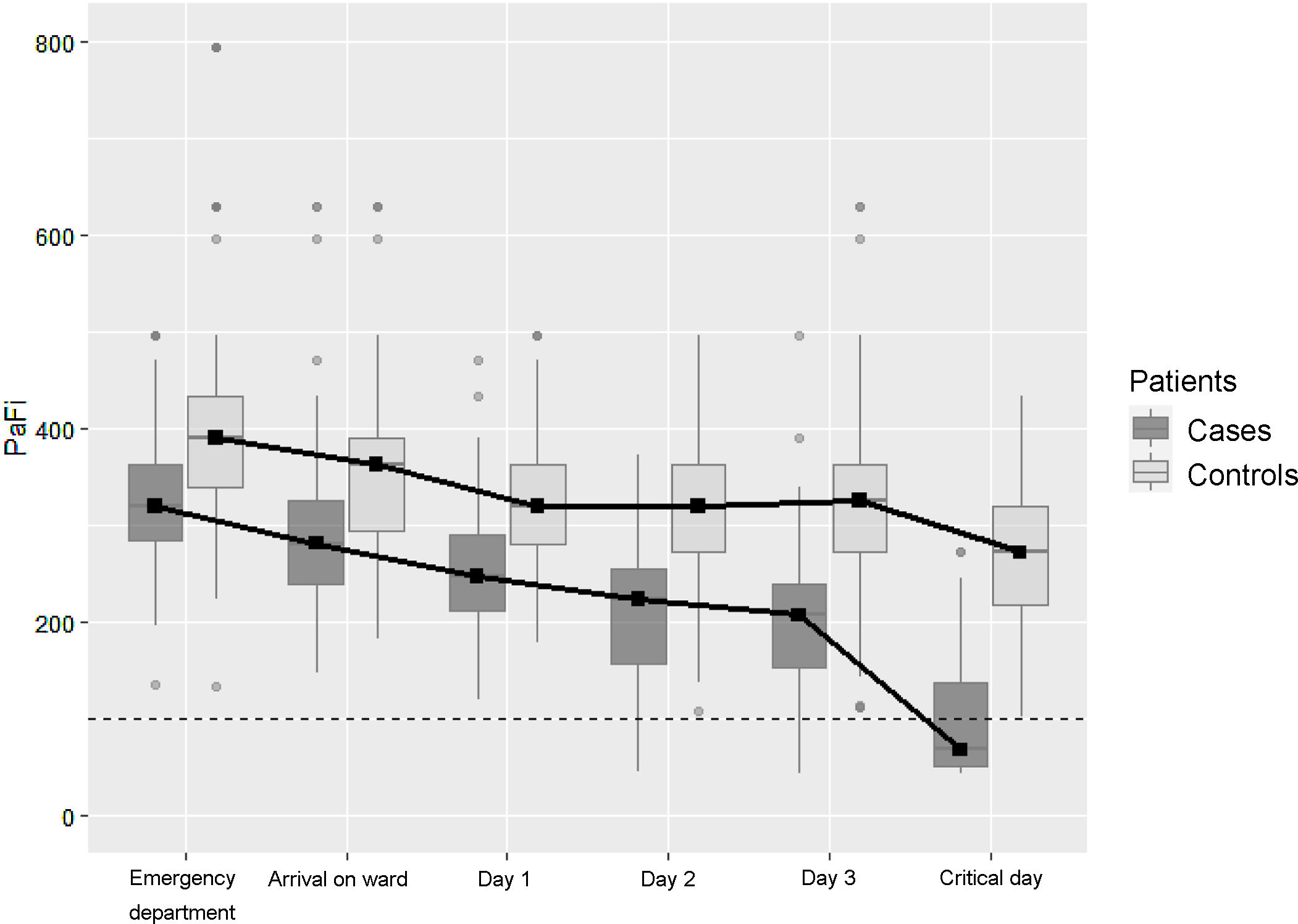
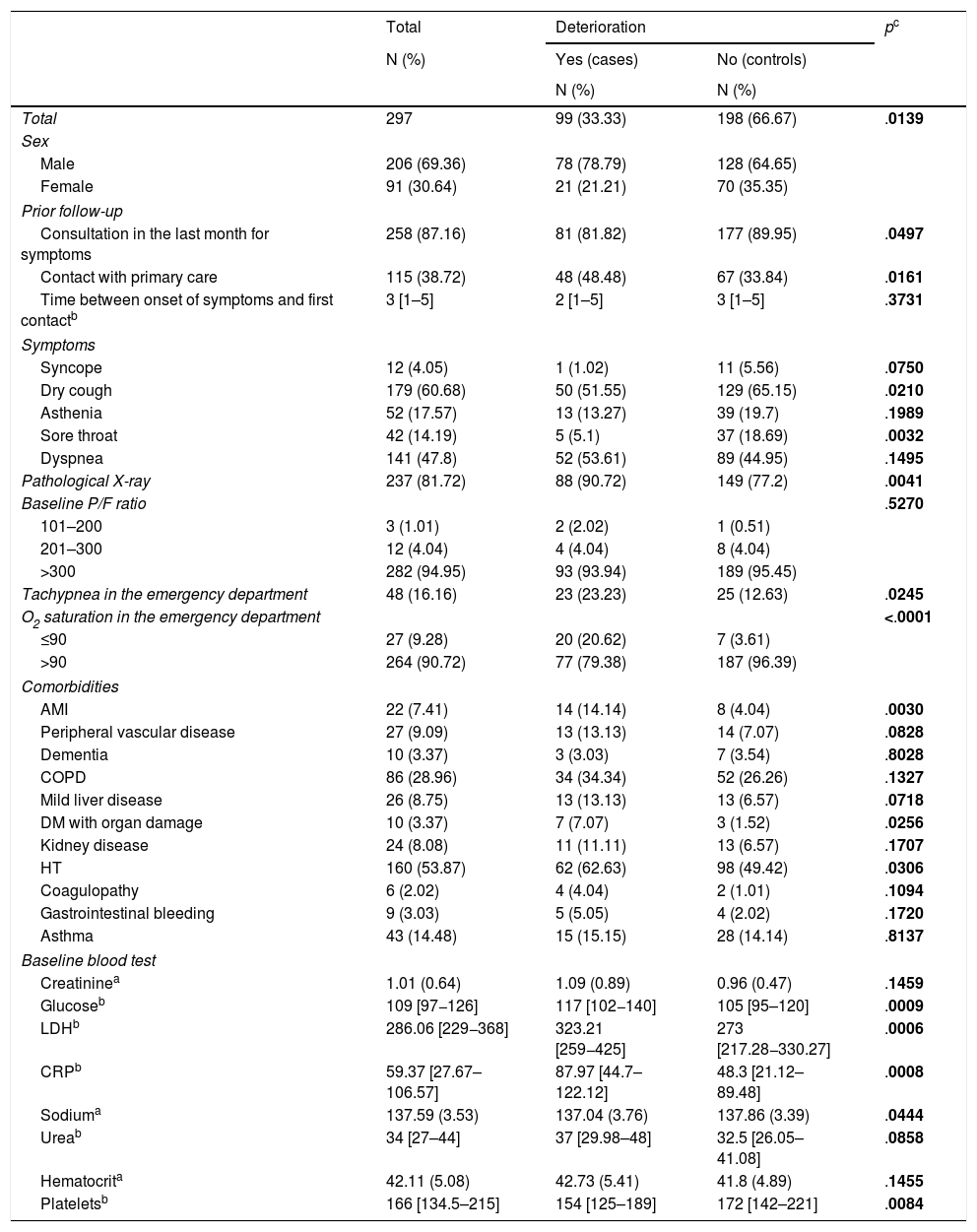
Methods designNested case-control study within a cohort. Setting: 13 acute care centers of the Osakidetza-Basque Health Service. Participants: patients hospitalized for COVID-19 with clinical deterioration—defined as onset of severe ARDS, ICU admission, or death—were considered cases. Two controls were matched to each case based on age. Sociodemographic data; comorbidities; baseline treatment; symptoms; date of onset; previous consultations; and clinical, analytical, and radiological variables were collected. An explanatory model of clinical deterioration was created by means of conditional logistic regression.
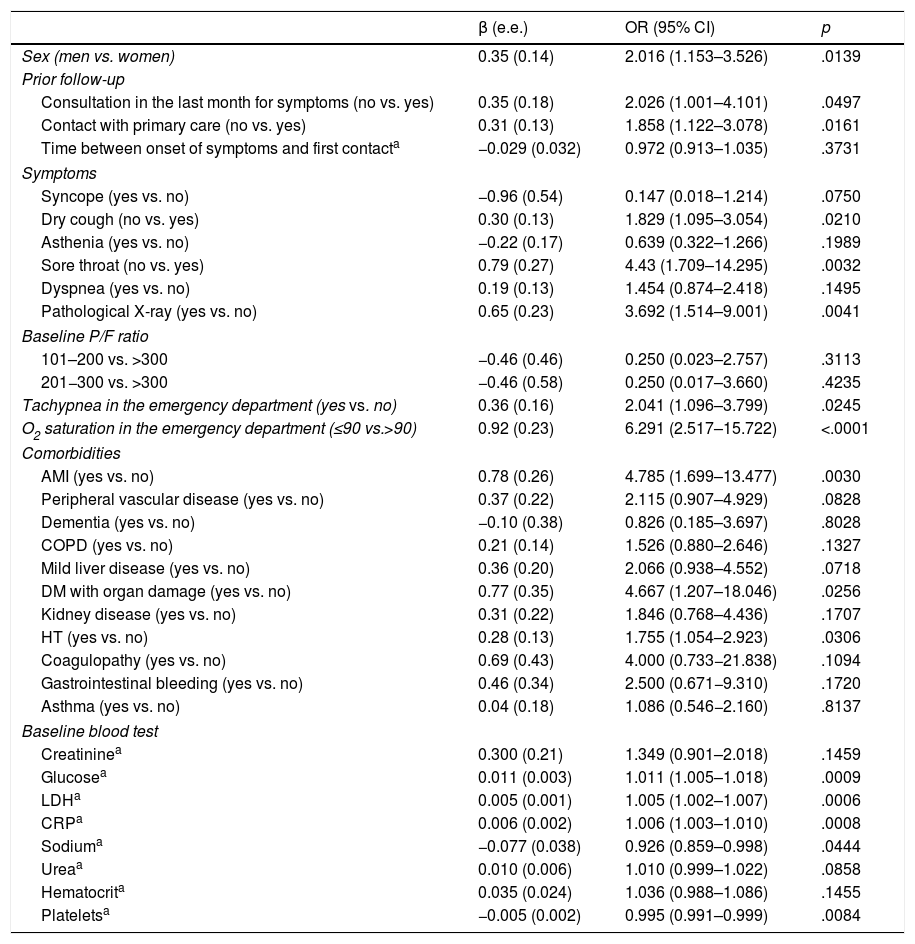
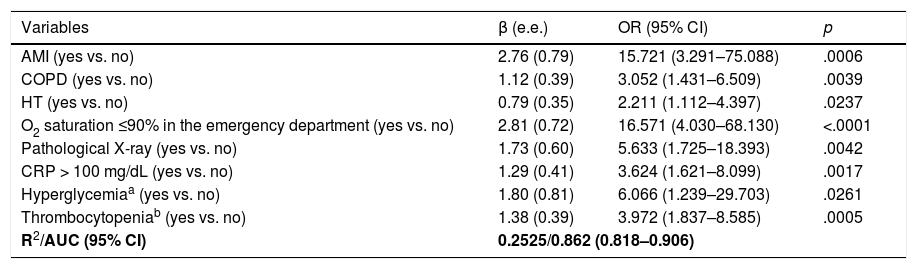
ResultsA total of 99 cases and 198 controls were included. According to the logistic regression analysis, the independent variables associated with clinical deterioration were: emergency department O2 saturation ≤90% (OR 16.6; 95%CI 4–68), pathological chest X-ray (OR 5.6; 95%CI 1.7–18.4), CRP > 100 mg/dL (OR 3.62; 95%CI 1.62–8), thrombocytopenia with <150,000 platelets (OR 4; 95%CI 1.84–8.6); and a medical history of acute myocardial infarction (OR 15.7; 95%CI, 3.29–75.09), COPD (OR 3.05; 95%CI 1.43–6.5), or HT (OR 2.21; 95%CI 1.11–4.4). The model’s AUC was 0.86. On the univariate analysis, female sex and presence of dry cough and sore throat were associated with better clinical progress, but were not found to be significant on the multivariate analysis.
ConclusionThe variables identified could be useful in clinical practice for the detection of patients at high risk of poor outcomes.
Existe controversia sobre los mejores factores predictores de deterioro clínico en la COVID-19.
ObjetivoIdentificar factores predictores de riesgo de deterioro en pacientes hospitalizados por COVID-19.
MétodosDiseño: caso-control anidado dentro de una cohorte. Ámbito: 13 centros de agudos de Osakidetza-Servicio Vasco de Salud. Participantes: se consideró casos a pacientes hospitalizados por COVID-19 con deterioro clínico, definido como la aparición de síndrome de distrés respiratorio del adulto grave, ingreso en UCI o fallecimiento. Se emparejaron 2 controles por caso en función de la edad. Se recogieron variables sociodemográficas, comorbilidades, tratamientos basales, síntomas y fecha de inicio, consultas previas, así como variables clínicas, analíticas y radiológicas. Se creó un modelo explicativo del deterioro clínico mediante regresión logística condicional.
ResultadosSe incluyeron 99 casos y 198 controles. Mediante análisis de regresión logística las variables independientes asociadas con deterioro clínico fueron: saturación de O2 en Urgencias ≤90% (OR = 16,6, IC del 95%, 4–68), radiografía de tórax patológica (OR = 5,6, IC del 95%, 1,7–18,4), PCR > 100 mg/dL (OR = 3,62, IC del 95% 1,62–8) y trombocitopenia < 150.000 plaquetas (OR = 4, IC del 95%, 1,84–8,6) y, entre los antecedentes, haber padecido infarto agudo de miocardio (OR = 15,7, IC del 95%, 3,29–75,09), EPOC (OR = 3,05, IC del 95%, 1,43–6,5) o hipertensión arterial (OR = 2,21, IC del 95%1,11–4,4). El área bajo la curva alcanzado por el modelo fue 0,86. En el análisis univariado, se asociaron con mejor evolución clínica el sexo femenino, la presencia de tos seca y dolor de garganta, pero no resultaron significativas en el análisis multivariado.
ConclusiónLas variables identificadas podrían ser de utilidad en la práctica clínica para la detección de pacientes con alto riesgo de mala evolución.
Article
Diríjase desde aquí a la web de la >>>FESEMI<<< e inicie sesión mediante el formulario que se encuentra en la barra superior, pulsando sobre el candado.

Una vez autentificado, en la misma web de FESEMI, en el menú superior, elija la opción deseada.

>>>FESEMI<<<