A high incidence of pulmonary embolism has been described during the coronavirus pandemic.
MethodsThis work is a single-center retrospective study which reviewed computed tomography pulmonary angiograms ordered due to suspected pulmonary embolism during two periods: from March 1, 2020 to May 31, 2020 (pandemic) and during the same interval in 2019 (control).
ResultsTwenty-two pulmonary embolisms were diagnosed during the control period and 99 in the pandemic, 74 of which were associated with COVID-19. Of all patients hospitalized with COVID-19, 5.3% had a pulmonary embolism, with a delay between the two diagnoses of 9.1 ± 8.4 days. During the pandemic, patients with pulmonary embolism had fewer predisposing conditions (previous pulmonary embolism 5.1 vs. 18.2%, p = .05; previous surgery 2 vs. 35.4%, p = .0001; deep vein thrombosis 11.1 vs. 45.5%, p = .0001); peripheral pulmonary embolisms were the most frequent (73.5 vs. 50%, p = . 029).
ConclusionsThere is an increased risk of having a pulmonary embolism during the SARS-CoV-2 pandemic, which affects patients with a different clinical profile and more often causes distal pulmonary embolisms.
Se ha descrito una elevada incidencia de tromboembolismo pulmonar (TEP) durante la pandemia por coronavirus.
MétodosEstudio retrospectivo unicéntrico, con revisión de las angiografías pulmonares por tomografía computarizada solicitadas por sospecha de tromboembolismo pulmonar durante dos períodos, del 01 de marzo del 2020 al 31de mayo del 2020 (pandemia), e igual intervalo en 2019 (control).
ResultadosSe diagnosticaron 22 tromboembolismos pulmonares durante el período control y 99 en el pandémico, 74 asociados con COVID-19. El 5,3% de los pacientes hospitalizados con COVID-19 sufrió un tromboembolismo pulmonar, con un retraso entre ambos diagnósticos de 9,1 ± 8,4 días. Durante la pandemia, los pacientes con tromboembolismo pulmonar tenían menos condiciones predisponentes (tromboembolismo pulmonar previo 5,1 vs. 18,2%, p = 0,05, cirugía previa 2 vs. 35,4%, p = 0,0001, trombosis venosa profunda 11,1 vs. 45,5%, p = 0,0001), y los tromboembolismos pulmonares periféricos eran más frecuentes (73,5 vs. 50%, p = 0,029).
ConclusionesExiste un riesgo incrementado de sufrir un TEP durante la pandemia por SARS-CoV-2, que afecta a pacientes con perfil clínico diferente y causa más frecuentemente TEP distales.
Although coronavirus disease 2019 (COVID-19) fundamentally manifests as atypical pneumonia and severe acute respiratory syndrome, it can affect other organs and systems.1 Thrombotic complications have been described in various areas,2 frequently as pulmonary embolism (PE),2–7 and PE series have been conducted in autopsies,3 intensive care units (ICU),2,4,6 and patients hospitalized in conventional wards.5–7
To the extent of our knowledge, neither the change in PE incidence in the general population during the pandemic nor the possible differential characteristics of the PE that occurred during this period have been investigated.
Population and methodsWe conducted a retrospective study using the radiology and electronic medical record archives of a university hospital in the Community of Madrid (Spain).
Two time periods were defined: the study period, from March 01 to May 31, 2020, and a control period, which was this same period in 2019. During these periods, all computed tomography pulmonary angiogram (CTPA) requests in which a clinical suspicion of PE was listed were identified.
The tests had been conducted using multidetector computed tomography (CT) (Brilliance; Philips, Eindhoven, the Netherlands) following the standard protocols. All were re-analyzed by two expert radiologists in order to detect intraluminal filling defects in the proximal or distal pulmonary arteries (segmental or subsegmental). The medical records of patients who were ultimately diagnosed with PE were retrieved and the predisposing clinical conditions included in the revised Geneva score8 were recorded along with D-dimer, determined by immunoturbidimetry (STA®-Liatest® D-Di Plus, Stago, Asnières-sur-Seine, France).9
During the study period, the results of a real-time polymerase chain reaction (RT-PCR) for SARS-CoV-2 and time since COVID-19 symptoms onset and suspicion of PE were recorded. The COVID-19 diagnosis was based on World Health Organization (WHO) criteria.10
The incidence of COVID-19 in the population served by the hospital was obtained by consulting public records.11 The χ2 test was used for qualitative variables (expressed as proportions). Means of the quantitative variables were compared using Spearman’s test (for nonrelated variables), expressed as mean ± standard deviation. A value of p < .05 was considered significant.
The relative risk of PE between both periods was calculated based on the number of PE detected and the population attended to in each period with a 95% confidence interval (CI). The data were analyzed with the IBM SPSS 22 program (Armonk, NY; USA ).
The study was approved by the hospital’s research ethics committee (OE33/2020), which exempted it from the need to obtain consent, given the study’s retrospective nature.
ResultsDuring the control period, a CTPA was ordered for suspicion of PE for 145 patients, 22 of which (15.2%) were ultimately diagnosed with PE. During the study period, 465 CTPA were ordered for suspicion of PE, 99 of which (21.3%) were confirmed and eight (8%) of which were in patients hospitalized in the ICU. A total of 74 had previously been diagnosed with COVID-19, with a mean period between the diagnoses of 9.1 ± 8.4 days.
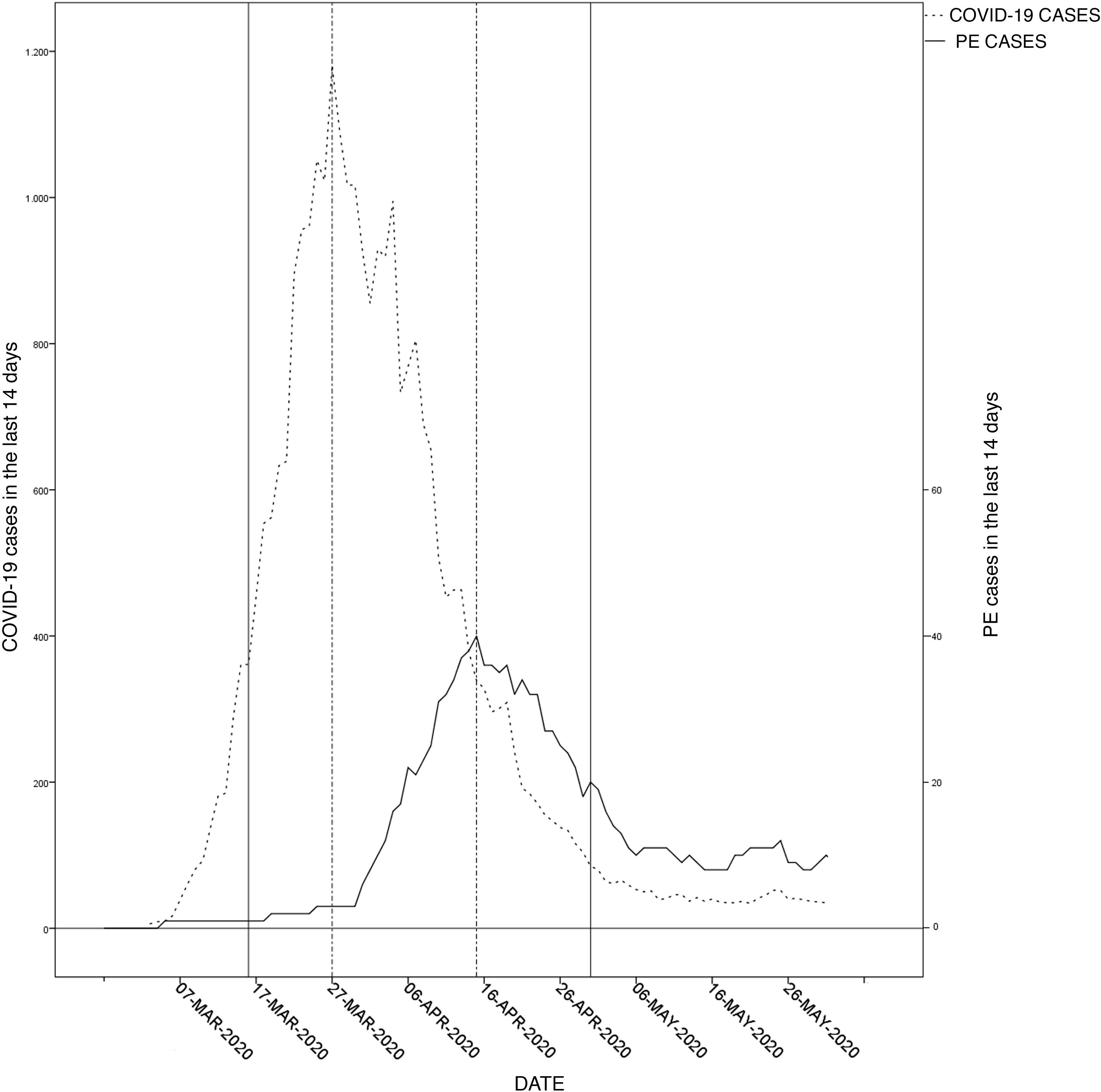
Of the 1292 patients hospitalized with a diagnosis of COVID-19 in this period, 5.7% (74) were diagnosed with PE. Considering the population served by the hospital, the relative risk of PE during the study period compared to the control period was 4.5 (95% CI 2.80–6.99). Fig. 1 shows the incidence curves for PE and COVID-19 estimated for the population served by the hospital. They had a similar trend, but PE occurred after a delay.
Graph of COVID-19 incidence and PE in the reference population during the pandemic. The lines represent the daily sum of cases during the previous 14 days for COVID-19 and for PE cases in the hospital’s reference population. The scale of PE incidence is ten times lower than that of COVID-19.
PE: pulmonary embolism.
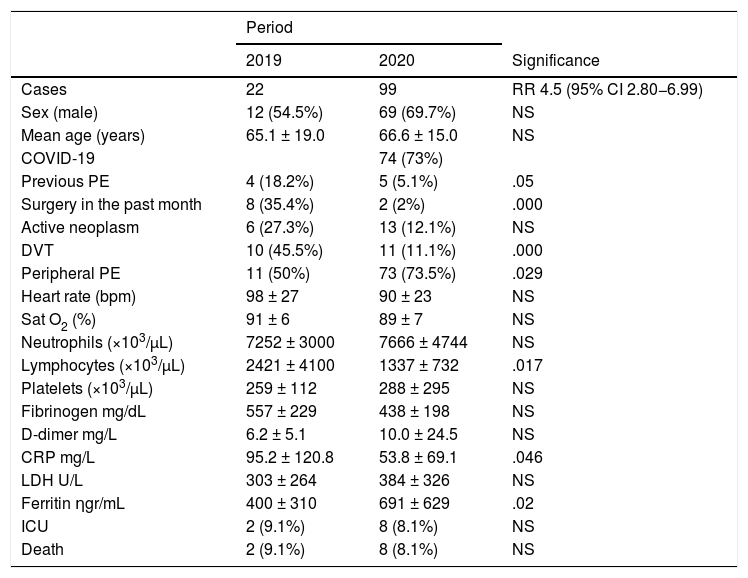
Table 1 summarizes the rest of the results. During the study period, patients with PE were more frequently men (69.7% vs. 54.5%, p = NS) and there were no relevant differences in mean age (65.1 ± 19.0 vs. 66.6 ± 15.0, p = NS). They had fewer episodes of prior PE (5.1% vs. 18.2%, p = .05), deep vein thrombosis (DVT) (11.1 vs. 45.5%, p = .0001), and surgery in the previous month (2% vs. 35.4%; p = .0001).
Clinical and radiological data of patients with a diagnosis of pulmonary embolism (PE) during the control period (2019) and the pandemic period (2020).
| Period | |||
|---|---|---|---|
| 2019 | 2020 | Significance | |
| Cases | 22 | 99 | RR 4.5 (95% CI 2.80−6.99) |
| Sex (male) | 12 (54.5%) | 69 (69.7%) | NS |
| Mean age (years) | 65.1 ± 19.0 | 66.6 ± 15.0 | NS |
| COVID-19 | 74 (73%) | ||
| Previous PE | 4 (18.2%) | 5 (5.1%) | .05 |
| Surgery in the past month | 8 (35.4%) | 2 (2%) | .000 |
| Active neoplasm | 6 (27.3%) | 13 (12.1%) | NS |
| DVT | 10 (45.5%) | 11 (11.1%) | .000 |
| Peripheral PE | 11 (50%) | 73 (73.5%) | .029 |
| Heart rate (bpm) | 98 ± 27 | 90 ± 23 | NS |
| Sat O2 (%) | 91 ± 6 | 89 ± 7 | NS |
| Neutrophils (×103/µL) | 7252 ± 3000 | 7666 ± 4744 | NS |
| Lymphocytes (×103/µL) | 2421 ± 4100 | 1337 ± 732 | .017 |
| Platelets (×103/µL) | 259 ± 112 | 288 ± 295 | NS |
| Fibrinogen mg/dL | 557 ± 229 | 438 ± 198 | NS |
| D-dimer mg/L | 6.2 ± 5.1 | 10.0 ± 24.5 | NS |
| CRP mg/L | 95.2 ± 120.8 | 53.8 ± 69.1 | .046 |
| LDH U/L | 303 ± 264 | 384 ± 326 | NS |
| Ferritin ηgr/mL | 400 ± 310 | 691 ± 629 | .02 |
| ICU | 2 (9.1%) | 8 (8.1%) | NS |
| Death | 2 (9.1%) | 8 (8.1%) | NS |
CI: confidence interval; LDH: lactate dehydrogenase; bpm: beats per minute; death: in-hospital death; CRP: C-reactive protein; RR: relative risk; RT-PCR: reverse-transcription polymerase chain reaction; Sat O2: oxygen saturation; DVT: deep vein thrombosis; ICU: intensive care unit admission; NS: not significant.
D-dimer levels were higher in cases of PE associated with COVID-19 (11.9 ± 27.5 vs. 3.4 ± 5.0 mg/L, p = .017) and they tended to be higher, though not significantly so, during the study period. PE were more frequently distal during the pandemic period (73.5% vs. 50%, p = .02) and also in patients with COVID-19 versus those who did not have COVID-19 (83.6% 57.9%; p = .005).
DiscussionOur study shows an increase in the incidence of PE during the first months of the pandemic, with up to 4.5 times greater risk compared to the same period the previous year. It includes PE that were associated and not associated with COVID-19 in hospitalization wards (91 cases) as well as in the ICU (eight cases). The difference between both periods (77 cases) almost entirely coincides with cases of PE associated with COVID-19 (74 episodes).
Some authors7 have described a cumulative incidence of PE of 24% in patients with COVID-19 in whom a CTPA is performed. This piece of data leads to confusion; it refers to the proportion of positive studies in patients who had the test performed for some reason and not the prevalence in the entire population hospitalized due to COVID-19.
Some meta-analyses have shown a high incidence of PE, but the majority of cases included come from patients hospitalized in the ICU, who have a greater incidence.12 Thus, the clearest information can be found in specific series. In a series of 184 patients with COVID-19 hospitalized in three ICUs, 25 (13.6%) had a PE,1 a similar figure (16.7%) to what was reported in a prospective analysis of 150 consecutive patients admitted in four other ICUs.13
On the other end, a series of patients hospitalized in the internal medicine ward of a hospital had a PE incidence of 6.2%,6 similar to our finding of 5.7%. Therefore, around 6% of patients hospitalized due to COVID-19 may have a PE, with figures increasing to 13%–17% in critical cases in the ICU.
We have observed a delay between COVID-19 diagnosis and suspicion of PE, which is clearly perceptible in Fig. 1. In light of the foregoing, we must be alert to signs of deterioration in COVID-19 patients after the first week of hospitalization.
PE found during the pandemic period differed from those diagnosed in the previous year. Patients had fewer predisposing conditions for having one and obstruction was more often distal. The same was true when comparing patients with PE with and without COVID-19. COVID-19 appears to be associated in and of itself with a greater risk of developing PE, probably greater than what is described in other types of lung pneumonia, in which distal PE is also more common.14
Other authors have also found an increase in distal PE in patients with COVID-196 and this could be at least in part due to the association of COVID-19 with in situ pulmonary thrombosis.15 An increase in other types of PE during the pandemic is in part to be expected, given that lockdown could be a cause of immobility and there has been a dramatic increase in the number of critically ill patients with greater risk of PE.
LimitationsThe retrospective nature of this study does not allow for standardization of suspicion of PE. A risk stratification was not conducted for the PE observed due to the small size of the sample. DVT may not have been systematically investigated during the peak of the pandemic due to logistical reasons. The majority of samples for RT-PCR were obtained using nasopharyngeal swabs, a technique with an estimated sensitivity of 63%.16 Therefore, some of the 25 cases of PE without a COVID-19 diagnosis could have been infected.
Estimated incidence of PE for the population is just an approximation, there were many possible causes for a loss of cases that could cause an underestimation, including patients who died in nursing homes and terminal cases which were not investigated. In any case, the real incidence of PE during the pandemic would be higher, not lower, than during the control period and would lend support to the initial hypothesis.
It is also plausible that CT scans were underused due to the emergency situation. Nevertheless, a CTPA was ordered due to suspicion of PE in a third of patients hospitalized due to COVID-19 during the study period, with a rate of confirmed diagnosis of 21.3%, which was not statistically different from the rate found during the control period.
When used correctly, CTPA should detect PE in 30% of all CTPAs ordered.17 Therefore, our data do not suggest underuse in any of the periods and are in agreement with the test’s diagnostic capacity in other studies conducted on patients with COVID-19.6
ConclusionsThe COVID-19 pandemic is associated with a greater risk of PE. This risk seems to increase one or two weeks following infection. During the pandemic period, PE affected patients with a different clinical profile who often had COVID-19. In addition, the obstruction of vessels was more frequently distal. Our results support the hypothesis that COVID-19 is a risk factor for having a PE with specific characteristics.
FundingThis research has not received specific grants from agencies in the public, commercial, or non-profit sectors.
Conflicts of interestThe authors declare that they do not have any conflicts of interest.
Please cite this article as: García-Lledó A, del Palacio-Salgado M, Álvarez-Sanz C, Pérez-Gil MM, Cruz-Díaz Á. Tromboembolismo pulmonar durante la pandemia por SARS-CoV-2: características clínicas y radiológicas. Rev Clin Esp. 2022;222:354–358.