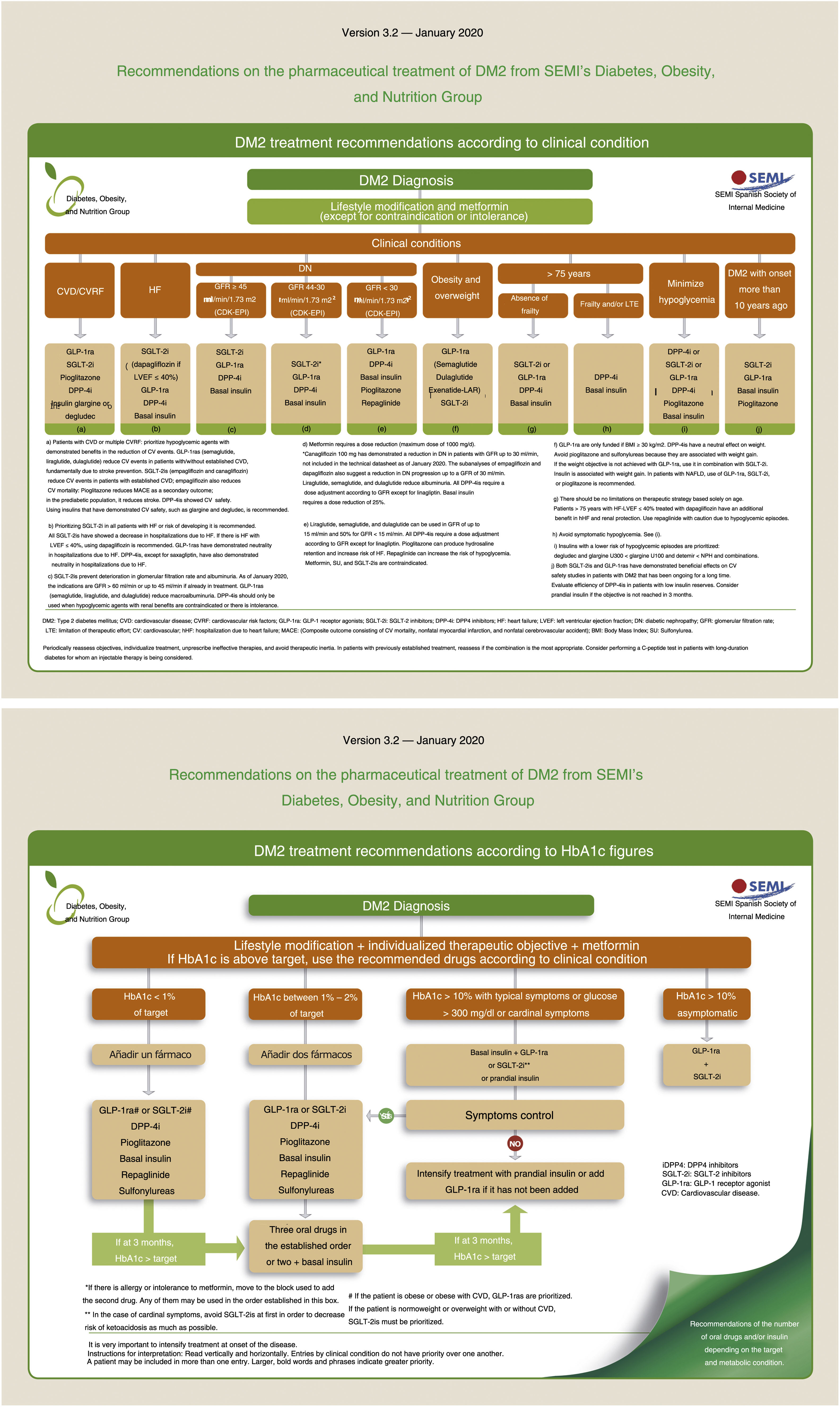
Type 2 diabetes is a big health concern due to its high prevalence and morbi-mortality. Medical treatment has a growing complexity which is focus on patients’ clinical situations. This article contains a consensus statement about recommendations on medical treatment of type-2 diabetes from the Working Group of Diabetes, Obesity and Nutrition of Spanish Society of Internal Medicine. The aim of this consensus is to facilitate therapeutic decision-making to improve the diabetes patients care. The document prioritizes treatments with cardiovascular, especially heart failure, and real benefits.
La diabetes tipo 2 constituye un problema de salud de elevada prevalencia y morbimortalidad. El tratamiento médico tiene una complejidad creciente en relación con las diversas situaciones clínicas del paciente. Este articulo recoge un documento de consenso de las recomendaciones para el tratamiento médico de la diabetes tipo 2 del Grupo de Diabetes, Obesidad y Nutrición de la Sociedad Española de Medicina Interna. El objetivo principal de este articulo es facilitar la toma de decisiones terapéuticas para mejorar la atención de los pacientes con diabetes. El documento prioriza los tratamientos con beneficios cardiovasculares, especialmente la insuficiencia cardíaca, y renales.
Article
Diríjase desde aquí a la web de la >>>FESEMI<<< e inicie sesión mediante el formulario que se encuentra en la barra superior, pulsando sobre el candado.

Una vez autentificado, en la misma web de FESEMI, en el menú superior, elija la opción deseada.

>>>FESEMI<<<