The SERPINA1 gene encodes the protein Alpha-1 Antitrypsin (AAT1). Possible imbalances between the concentrations of proteases and antiproteases (AAT1) can lead to the development of serious pulmonary and extrapulmonary pathologies. In this work we study the importance of this possible imbalance in patients with COVID-19.
ObjectivesTo correlate the severity of the symptoms of SARS-COV-2 infection with the AAT1 concentrations at diagnosis of the disease.
MethodsAn observational, prospective, cross-sectional, non-interventional, analytical study was carried out where 181 cases with COVID-19 admitted to the “Lozano Blesa” University Clinical Hospital of Zaragoza were selected. The concentration of AAT1 was studied in all of them and this was correlated with the clinical aspects and biochemical parameters at hospital admission.
Results141 cases corresponded to patients with severe COVID and 40 patients with mild COVID. AAT1 levels were positively correlated with the days of hospitalization, severity, C-Reactive Protein, ferritin, admission to Intensive Care, and death, and presented a negative correlation with the number of lymphocytes/mm3. AAT1 concentrations higher than 237.5 mg/dL allowed the patient to be classified as “severe” (S72%; E78%) and 311.5 mg/dL were associated with the risk of admission to Intensive Care or Exitus (S67%; E79%).
ConclusionsLevels of the SERPINA1 gene expression product, AAT1, correlate with the severity of COVID-19 patients at diagnosis of the disease, being useful as a prognostic biomarker.
El gen SERPINA1 codifica la proteína Alfa-1 Antitripsina (AAT1). Los posibles desequilibrios entre las concentraciones de proteasas y antiproteasas (AAT1) puede hacer que se desarrollen patologías pulmonares y extrapulmonares graves. En este trabajo estudiamos la importancia de este posible disbalance en los pacientes con COVID-19.
ObjetivosCorrelacionar la gravedad de la clínica de la infección por SARS-COV-2 con las concentraciones de AAT1 al diagnóstico de la enfermedad.
MétodosSe ha llevado a cabo un estudio observacional, prospectivo, transversal, no intervencionista con carácter analítico donde se seleccionaron 181 casos con COVID-19 ingresados en el Hospital Clínico Universitario “Lozano Blesa” de Zaragoza. A todos ellos se les estudió la concentración de AAT1 y ésta se correlacionó con los aspectos clínicos y parámetros bioquímicos al ingreso hospitalario.
Resultados141 casos correspondían a pacientes con COVID grave y 40 pacientes con COVID leve. Los niveles de AAT1 se correlacionaron positivamente con los días de estancia, la gravedad, la Proteína C Reactiva, la ferritina, el ingreso en Cuidados Intensivos y el fallecimiento y presentaron una correlación negativa con el número de linfocitos/mm3. Las concentraciones de AAT1 superiores a 237,5 mg/dL permitieron catalogar al paciente como “grave” (S72%; E78%) y de 311,5 mg/dL se asociaron con el riesgo de ingreso en Cuidados Intensivos o Exitus (S67%; E79%).
ConclusionesLos niveles del producto de la expresión del gen SERPINA1, la AAT1, se correlacionan con la gravedad de los pacientes COVID-19 al diagnóstico de la enfermedad siendo útil como biomarcador pronóstico.
Coronavirus was the cause of a high number of deaths in the first months of its appearance. According to official figures, the COVID-19 pandemic caused 120,715 deaths and the number of cases of COVID-19 who have been confirmed in Spain is 13,825,052.1
In the scientific community there is no consensus about the pathophysiology of this disease. At present, the most accepted pathophysiological model is the one that distinguishes 3 phases depending on the time of evolution of the disease: phase 1 or viral infection, phase 2 or pulmonary infection, and phase 3 or inflammatory activity. But even after the pandemic is declared over, the pathology of SARS-COV-2 continues to be studied, since the aforementioned model leaves important questions about the evolution of the disease unanswered.
From a pathophysiological point of view, it is known that2: 1) SARS-COV-2 is an RNA virus, 2) it interacts with the ACE2 receptor to be able to enter the cell it infects, 3) it produces an important activation of acute phase reactants such as C-reactive protein, Ferritin and IL-6 and 4) it causes a severe prothrombotic state.
From the above we can deduce that one of the target cells involved in this pathophysiological model is the macrophage, which is closely related not only to IL-6 but also to iron metabolism.3,4 Today, this disease coexists in society in a more controlled way, despite the fact that the management and treatment of these patients continues to be a challenge; since there is no pathophysiological model nor a good biomarker that predicts the evolution of patients.
Alpha-1 Antitrypsin (AAT1) is an acute phase glycoprotein, water-soluble and diffusible in tissues. It has a molecular weight of 52-kDa and a half-life in blood of about 5 days.5 At baseline, the body produces 34 mg/kg/day, an activity that is translated into plasma concentrations of around 100–200 mg/dL, being the most abundant antiproteinase in serum.6 AAT1 is encoded by the SERPINA1 gene, which is located on chromosome 14q32.1 and comprises four coding exons (II–V), three untranslated exons (Ia–Ic), and six introns.7
The synthesis of AAT1 is carried out mainly in hepatocytes (≈80%), and it is a multifactorial protein, with an important serine protease inhibitory property in human serum. The specific substrate of AAT1 is neutrophil elastase (synonyms: elastase 2, ELA2), with which it reacts with an association constant of the highest known in physiology. The mechanism of action of AAT1 is based on the amino acids methionine and serine, located in the mobile zone of AAT1; These form covalent bonds with neutrophilic elastase, inhibiting it and facilitating its phagocytosis by macrophages, for its subsequent degradation in the hepatocyte.8–10 The primary site of AAT1 activity is in the lung, protecting the fragile connective tissue of the lower respiratory tract from uncontrolled proteolysis caused by neutrophils during inflammation.11
The AAT1 gene is activated mainly by products generated in inflammatory processes. The best known are bacterial endotoxin or lipopolysaccharide (LPS); interferon-α; the cytokines IL-1, IL-6 and TNF-α; and various end products of nitrooxidative stress generically called AGEs (Advanced Glycation End products). Being an acute phase reactant, its plasma levels increase rapidly in response to inflammatory or infectious stimuli, between 2–4 times; accompanying C-reactive protein (CRP) and amyloid A, and this increase is maintained between 7 and 15 days.
In SARS-COV-2 infection, hypoxia, inflammation and elevation of CRP, Ferritin and IL-6 have been demonstrated in severe patients. Due to the close relationship of the macrophage and neutrophil with CRP and iron metabolism, which are highly altered in severely ill patients affected by COVID-19, we deduce that neutrophil activation contributes to the pathophysiology of this entity.12 For this reason we have investigated whether AAT1 activity could be a marker of the serious inflammatory process that occurs.13 Therefore, the need arises to investigate whether the SERPINA1 gene and its expression are altered in this serious entity.
MethodsAn observational, prospective, cross-sectional, non-interventional study with an analytical nature has been carried out. As the object of this study, 181 cases with SARS-COV-2 were selected during the months of September 2020 to January 2021. All of them perfectly characterized from a clinical point of view, from the Internal Medicine and Emergency Services of the Clinical Hospital University “Lozano Blesa” of Zaragoza. The inclusion criteria in the study were the following: 1) age over 18 years, 2) diagnosis of SARS-COV-2 infection at the time of blood collection used as material for the study, 3) diagnostic confirmation of SARS-COV-2 infection through polymerase chain reaction tests and/or antigen tests, 4) signing the informed consent and 5) agreeing with the conduct and purpose of the study.
The study was approved by the Ethics and Research Committee of Aragon (CEICA) and was developed in accordance with the principles of the Declaration of Helsinki. After signing the informed consent form, a series of clinical, biochemical and genetic parameters were collected for each patient at the time of hospital admission and on subsequent days. The variables collected were the following: 1) Age, gender, severity and number of days of hospital stay, 2) biochemical data: ferritin, interleukin 6, lymphocytes and C-reactive protein at diagnosis of the disease or at hospital admission, if applicable, 3) thorax radiological rale score (SARS-COV-2 CXR), 4) need for admission to the intensive care unit and death and 5) serum concentration of AAT1 at diagnosis of the disease or at hospital admission, if applicable.
In this study we considered patients with “mild COVID” those who attended the Emergency Department, were diagnosed and treated, and were discharged home that same day. On the other hand, we call “severe COVID” the disease suffered by those patients who required hospital admission and, within these, we differentiate “critical COVID” as the ones that required admission to the Intensive Care Unit (ICU). The statistical analysis was carried out using the SPSS-PC 29.0 statistical package, first carrying out a descriptive study and then the different variables were analyzed together considering that there was a statistically significant relationship with a value of p < 0.05.
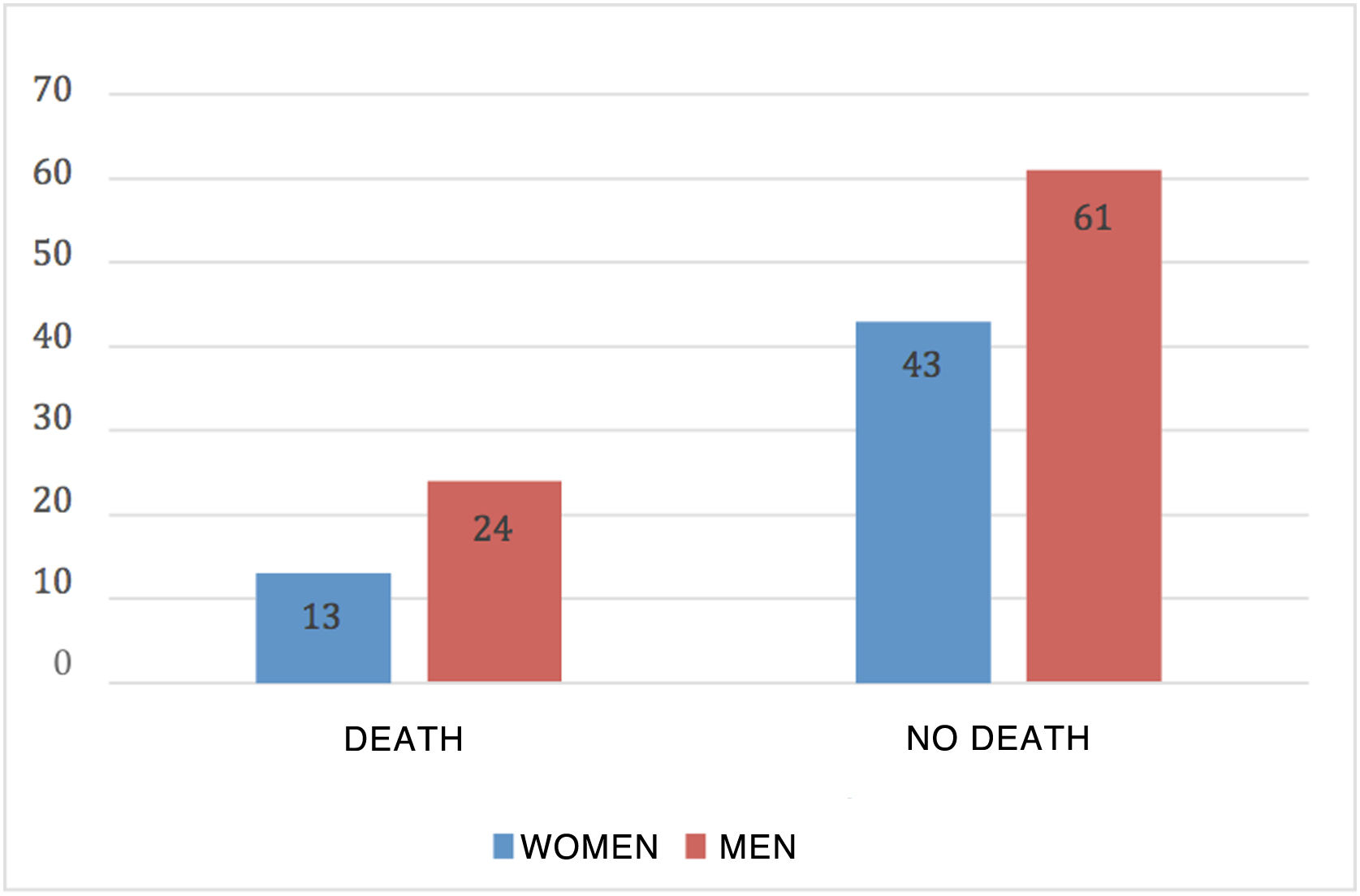
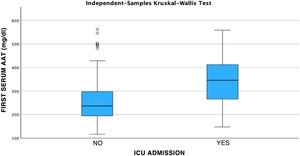
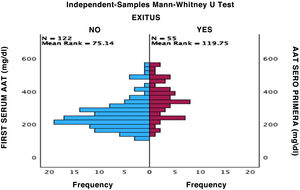
ResultsOf the 181 patients who were selected based on the previously described criteria, 141 cases corresponded to patients with severe COVID and 40 patients to mild COVID. After the classification of patients based on their severity, previously explained in the Material and Methods section, it was observed that of the cases with mild COVID, 50% were men and 50% women. Severe COVID had a higher prevalence in men, accounting for 60.28% of the total cases analyzed. Of the patients admitted, 41.13% required care in the ICU, of which 67.24% were men and 32.76% women. The mean serum AAT1 concentrations were 286.31 mg/dL (291.37 mg/dL in men and 297.99 mg/dL in women). Patients admitted to the conventional ward had mean concentrations of 270.76 mg/dL and those admitted to the ICU 359.92 mg/dL.
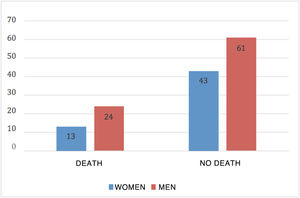
Mortality in patients with severe COVID was 26.42%; 64.86% were men and 35.14% were women (Fig. 1).
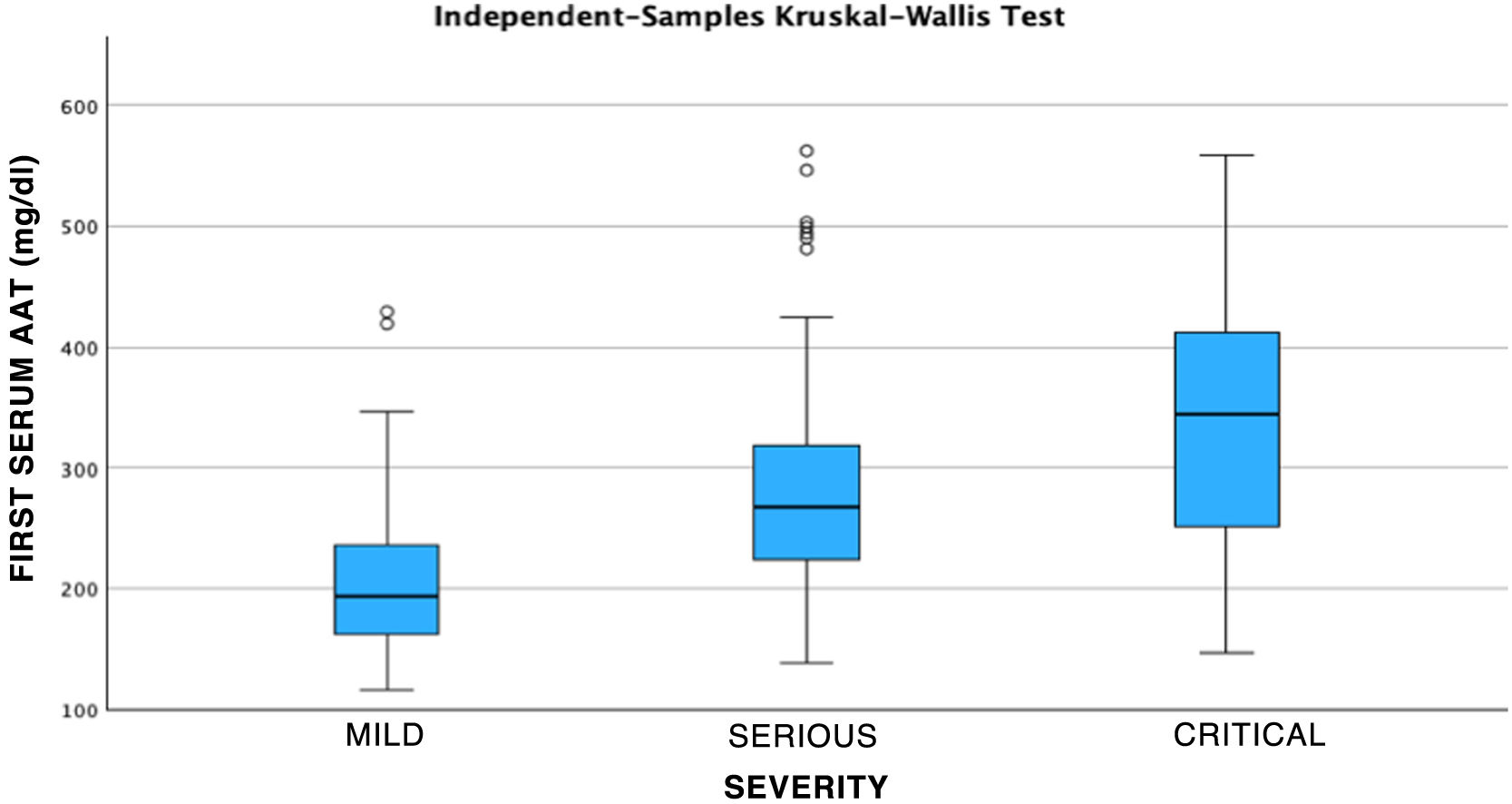
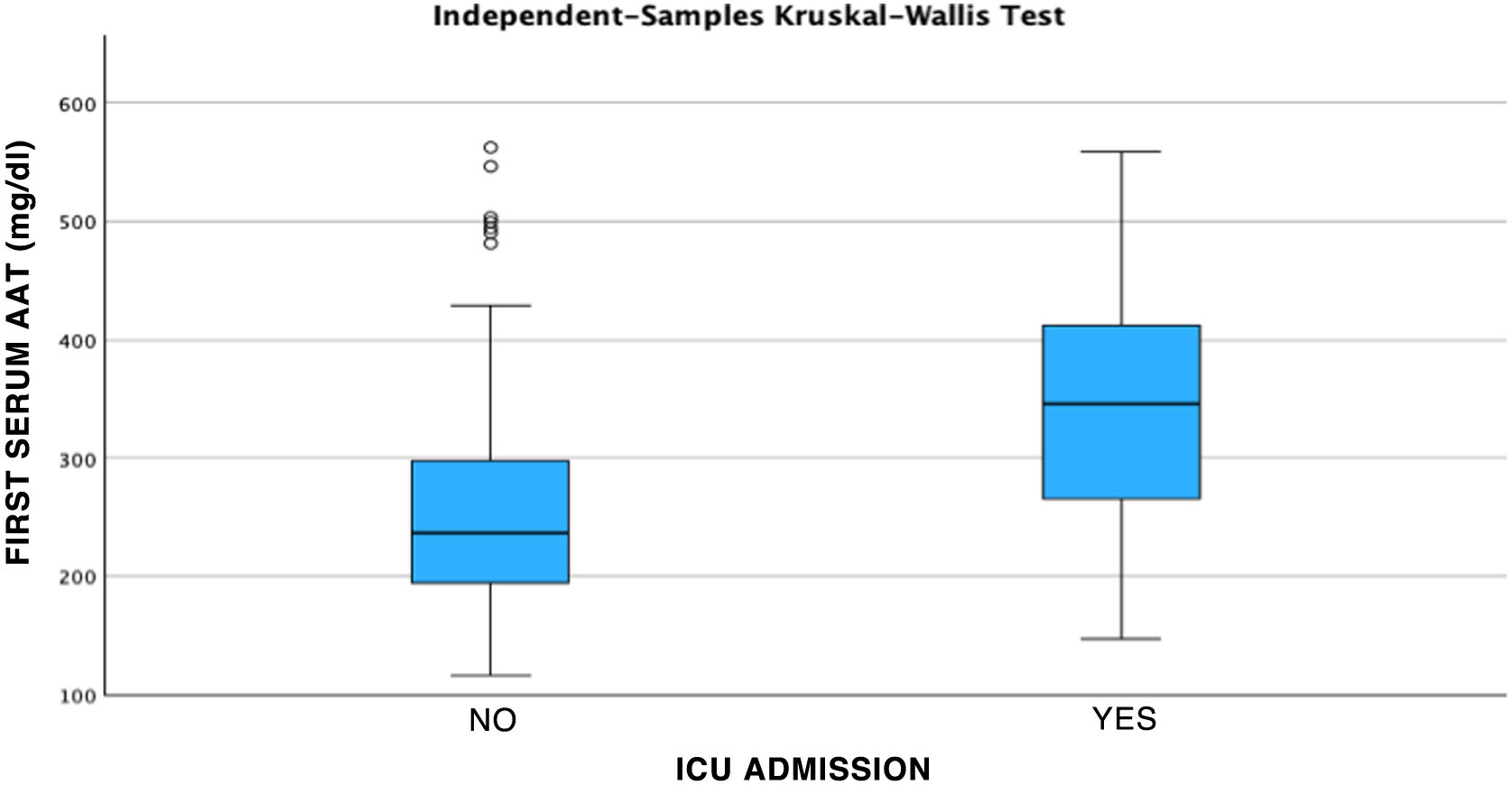
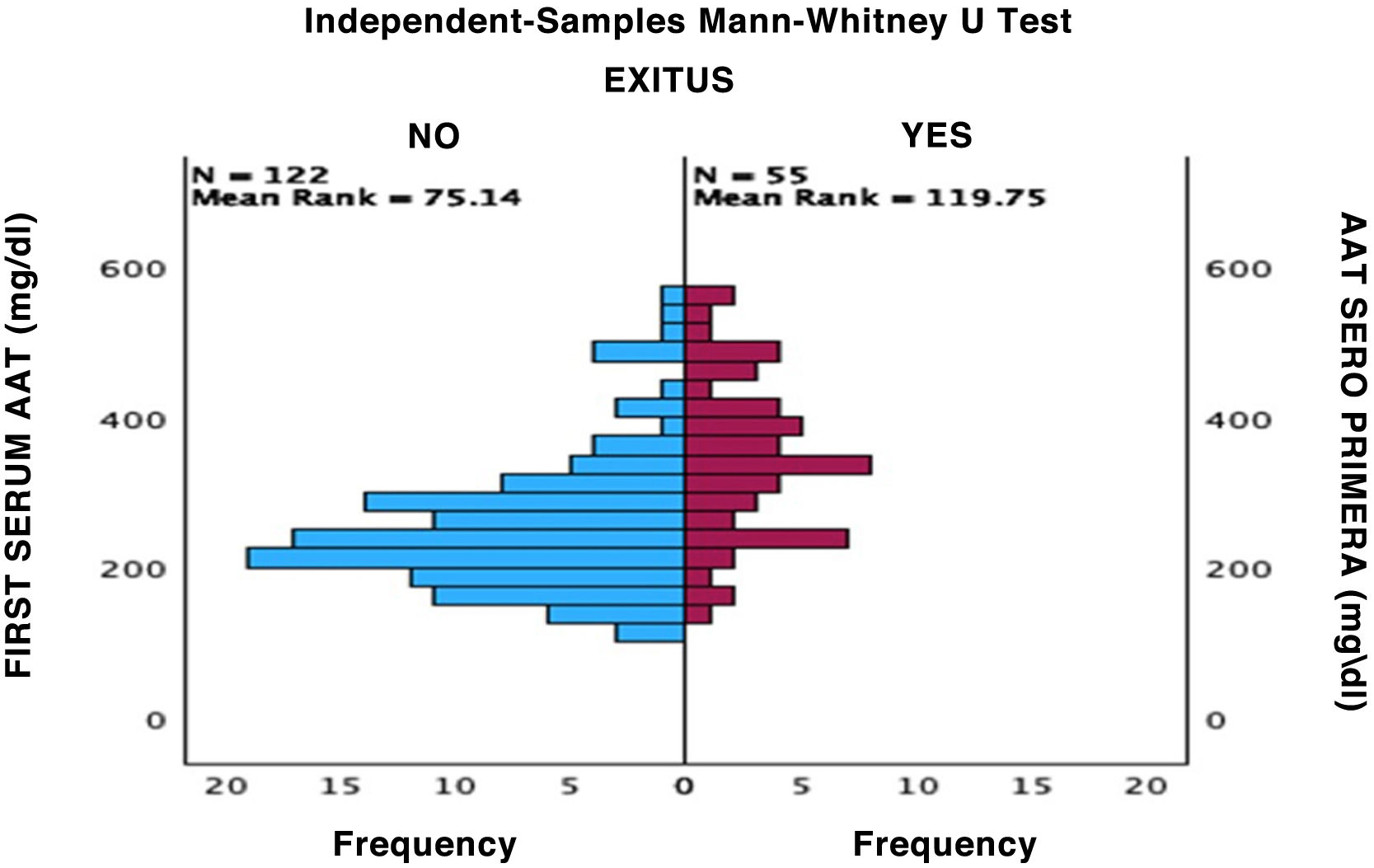
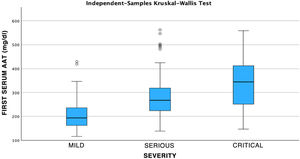
After performing the linear regression analysis between the different quantitative variables, only a moderate correlation was found (r = 0.49, p < 0.05) between AAT1 concentrations at admission and the days of stay, the rest being weak or very weak correlations (p < 0.05). Subsequently, the analysis was carried out using the Chi-square test of the relationship between the qualitative variables, being statistically significant (p < 0.05) the correlation between the severity of the patient (referring to the patient who requires hospital admission), admission to ICU (the patient being by definition “critical”) and the possible death of the patient. None of these variables was related to the patient’s sex. Next, we analyzed the correlation between the different biomarkers and the severity of each patient, the sex, and the risk of admission to the ICU or death. Statistically significant relationships were found between the concentrations of AAT1 at hospital admission with the number of lymphocytes (in this case the correlation was negative), the values of C-reactive protein and ferritin, and the severity, admission to the ICU, or death of the patient. IL-6 had no correlation with any of the previously mentioned qualitative variables (Figs. 2, 3 and 4).
There was not a correlation found between the variables analyzed and the sex of each patient. A clear correlation of the biomarkers with the radiological score was also not found.
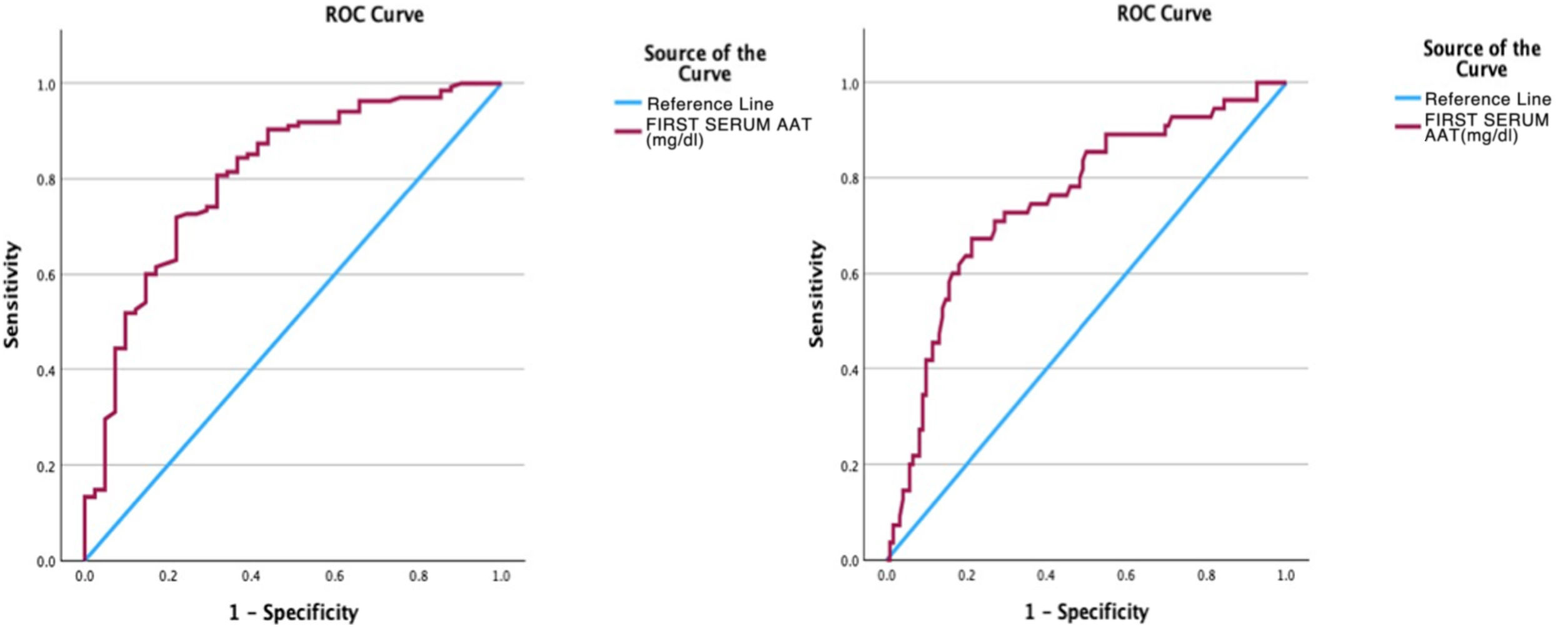
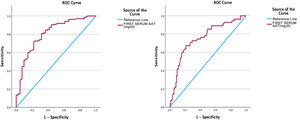
Subsequently, an analysis of the AAT1 was carried out using the ROC curve as a test with prognostic value at hospital admission in relation to the severity of the patient, the risk of admission to the ICU and the risk of death, obtaining the following results in the form of graphs and dots cutoff for each of the events or severity (Fig. 5).
- •
In this way, the area under the curve relating the AAT1 concentration on admission to the patient’s severity was 0.806 (95% CI 0.73–0.88), obtaining a cut-off point for AAT1 regarding the concept “severe patient” of 237.5 mg/dL, with an S of 71.9% and an E of 78%.
- •
Regarding the ROC curve obtained relating AAT1 activity to hospital admission and risk of admission to the ICU or death, the area under the curve was 0.75 (95% CI 0.67–0.83), obtaining a cut-off point for AAT1 of 311.5 mg/dL, with an S of 67.3% and an E of 78.7%.
Our study concluded with the identification of a gene whose expression is closely involved in the pathophysiology of the organ dysfunction that occurs in patients with severe SARS-COV-2 infection. The study of the expression of the SERPINA1 gene in our cohort has provided a large amount of information that other molecules such as IL-6 have not generated, which is one of the most studied in this entity.14 The SERPINA1 gene is highly activated in this syndrome and the study of its expression product is simple, fast, cheap and reproducible.
In COVID-19, both genetic and environmental factors may be involved in comorbidity.15 The genetic predispositions described in genes such as ACE1 or SERPINA1 may be related to this entity and curiously, the polymorphisms in these genes show frequencies that differ significantly between Westerners and East Asians16 and may contribute to the geographical disparities found regarding the severity and mortality of infection with the SARS-COV-2 virus.17 For all these reasons, we want to highlight that the results obtained in our environment provide very important data for understanding the pathobiology of COVID-19 and constitute the basis for the development of future lines of research.
To date, few studies17,18 have been carried out in the COVID-19 population that involve the SERPINA1 gene, but these have implicated AAT1 1) in the inhibition of IL-1B (which induces lung damage by stimulating the production of IL-6 and TNF-α), 2) to antagonize thrombotic complications caused by increased thrombin and plasmin, 3) to inhibit IL-6 concentrations, 4) to inhibit IL-8 (closely related to neutrophilic activation and acute lung damage, 5) to inactivate the transmembrane protease serine 2 of the host cell receptors (TMPRSS2), 6) to limit the uptake of SARS-COV2 viral particles, 7) to favor the differentiation of T lymphocytes and activated M2 macrophages, 8) to protect from the activation of the caspase 3 pathway (and therefore oxidative stress), 9) to suppress the production of TGF-B (closely related to irreversible fibrosis) and 10) to reduce the production of superoxide radicals released by activated neutrophils, as well as neutrophilic migration. This is why our results are truly relevant since they implicate AAT1 with the severity and phenotype of the patients, such that the higher the AAT1, the worse the prognosis and the greater the probability of admission to the ICU or death.
However, methodologically we must mention limitations since we have not studied the genotype of the SERPINA1 gene and the patients have not been classified according to certain risk factors that influence not only the acute phase of the disease but also the respiratory functional situation and quality of residual life once the acute phase is over.19 On the other hand, and given that IL-6 concentrations did not correlate with any of the variables studied at hospital admission, we did not take into account the AAT1/IL-6 ratio, whose increased value protects COVID-19 patients from developing of severe disease and thrombosis as demonstrated by Philippe A et al.20 Comparing this French work with ours, we found significant differences in the cut-off points for severely ill patients (157 mg/dL) and critically ill patients (230 mg/dL), with our values being significantly higher at 237.5 and 311.5 mg/dL. respectively.
Unfortunately, the study of the SERPINA1 gene in COVID-19 does not solve the problem of genotype/phenotype correlation. However, it has allowed us to classify the group of patients into “unforeseen, serious or critical phenotypes” with the practical implications from the healthcare point of view that this has entailed.
Finally, we must mention that, based on our study, and in support of the previous literature, it is possible to find ourselves facing a future therapeutic target in patients with severe or critical COVID-19: the exogenous administration of AAT1.21,22
ConclusionsPatients with severe COVID-19 present elevations in AAT1 compared to the basal plasma concentrations known in the healthy population.
The study of AAT1 concentration has proven to be useful as a prognostic biomarker in seriously ill patients affected by SARS-COV-2.
The phenotype of patients with SARS-COV-2 correlates with the AAT1 concentrations detected.
Conflict of interestsAll authors of this study declare the absence of conflict of interest.
FundingThis study has received funding from the Aragon Health Research Institute, Spain.